Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients
- PMID: 21703938
- PMCID: PMC3164390
- DOI: 10.1016/j.jpain.2011.04.008
Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients
Abstract
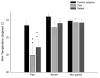
Many frontline chemotherapeutic agents produce robust neuropathy as a dose-limiting side effect; however, the persistence of chemotherapy-related sensory disturbances and pain are not well documented. We have previously investigated the qualities of bortezomib-induced pain, and now seek to determine the ongoing nature of this pain. Twenty-six control subjects and 11 patients who had previously been treated with bortezomib and who were experiencing ongoing pain consented to recurring quantitative sensory testing. A pilot immunohistochemistry study of skin innervation was also performed on patient-obtained biopsies. Psychophysical testing in patients revealed persistent changes including decreased skin temperature in the area of pain, diminished touch and sharpness detection, increased pegboard completion times, and decreased sensitivity to skin heating. Additionally, the intensity of pain, as captured by the use of a visual analog scale and pain descriptors, was reported by patients to be unchanged during the retest despite similar morphine equivalent daily doses. The patient skin biopsies displayed a marked decrease in the density of epidermal nerve fibers and Meissner's corpuscles. These results signify a persistent and severe impairment of Aβ, Aδ, and C fibers in patients with chronic bortezomib-induced chemoneuropathy. Further, this study reports a loss of both epidermal nerve fibers and Meissner's corpuscles.
Perspective: The results of this article indicate a persistent, painful peripheral neuropathy in patients treated with bortezomib. Pilot data indicates a loss of nerve fibers innervating the area of pain. This is the first paper to address the persistence, and potential contributing factors, of bortezomib chemoneuropathy.
Copyright © 2011 American Pain Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Basu S, Sodhi A. Increased release of interleukin-1 and tumour necrosis factor by interleukin-2-induced lymphokine-activated killer cells in the presence of cisplatin and FK-565. Immunol Cell Biol. 1992;70 ( Pt 1):15–24. - PubMed
-
- Benn T, Halfpenny C, Scolding N. Glial cells as targets for cytotoxic immune mediators. GLIA. 2001;36:200–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

