Replication of ribonucleotide-containing DNA templates by yeast replicative polymerases
- PMID: 21703943
- PMCID: PMC3147116
- DOI: 10.1016/j.dnarep.2011.05.009
Replication of ribonucleotide-containing DNA templates by yeast replicative polymerases
Abstract
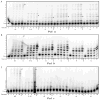
The major replicative DNA polymerases of S. cerevisiae (Pols α, δ, and ɛ) incorporate substantial numbers of ribonucleotides into DNA during DNA synthesis. When these ribonucleotides are not removed in vivo, they reside in the template strand used for the next round of replication and could potentially reduce replication efficiency and fidelity. To examine if the presence of ribonucleotides in a DNA template impede DNA synthesis, we determined the efficiency with which Pols α, δ, and ɛ copy DNA templates containing a single ribonucleotide. All three polymerases can replicate past ribonucleotides. Relative to all-DNA templates, bypass of ribo-containing templates is slightly reduced, to extents that depend on the identity of the ribo and the sequence context in which it resides. Bypass efficiencies for Pols δ and ɛ were increased by increasing the dNTP concentrations to those induced by cellular stress, and in the case of Pol ɛ, by inactivating the 3'-exonuclease activity. Overall, ribonucleotide bypass efficiencies are comparable to, and usually exceed, those for the common oxidative stress-induced lesion 8-oxo-guanine.
Published by Elsevier B.V.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Kornberg A, Baker T. DNA replication. 2. WH Freeman and Co; New York: 1992.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

