Distinctive genetic and clinical features of CMT4J: a severe neuropathy caused by mutations in the PI(3,5)P₂ phosphatase FIG4
- PMID: 21705420
- PMCID: PMC3122378
- DOI: 10.1093/brain/awr148
Distinctive genetic and clinical features of CMT4J: a severe neuropathy caused by mutations in the PI(3,5)P₂ phosphatase FIG4
Abstract
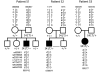
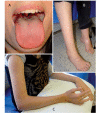
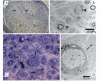
Charcot-Marie-Tooth disease is a genetically heterogeneous group of motor and sensory neuropathies associated with mutations in more than 30 genes. Charcot-Marie-Tooth disease type 4J (OMIM 611228) is a recessive, potentially severe form of the disease caused by mutations of the lipid phosphatase FIG4. We provide a more complete view of the features of this disorder by describing 11 previously unreported patients with Charcot-Marie-Tooth disease type 4J. Three patients were identified from a small cohort selected for screening because of their early onset disease and progressive proximal as well as distal weakness. Eight patients were identified by large-scale exon sequencing of an unselected group of 4000 patients with Charcot-Marie-Tooth disease. In addition, 34 new FIG4 variants were detected. Ten of the new CMT4J cases have the compound heterozygous genotype FIG4(I41T/null) described in the original four families, while one has the novel genotype FIG4(L17P/nul)(l). The population frequency of the I41T allele was found to be 0.001 by genotyping 5769 Northern European controls. Thirty four new variants of FIG4 were identified. The severity of Charcot-Marie-Tooth disease type 4J ranges from mild clinical signs to severe disability requiring the use of a wheelchair. Both mild and severe forms have been seen in patients with the same genotype. The results demonstrate that Charcot-Marie-Tooth disease type 4J is characterized by highly variable onset and severity, proximal as well as distal and asymmetric muscle weakness, electromyography demonstrating denervation in proximal and distal muscles, and frequent progression to severe amyotrophy. FIG4 mutations should be considered in Charcot-Marie-Tooth patients with these characteristics, especially if found in combination with sporadic or recessive inheritance, childhood onset and a phase of rapid progression.
Figures


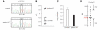
References
-
- Barisic N, Claeys KG, Sirotkovic-Skerlev M, Lofgren A, Nelis E, De Jonghe P, et al. Charcot-Marie-Tooth disease: a clinico-genetic confrontation. Ann Hum Genet. 2008;72:416–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH061675/MH/NIMH NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- R01 MH067257/MH/NIMH NIH HHS/United States
- R01 MH060870/MH/NIMH NIH HHS/United States
- R01 MH059586/MH/NIMH NIH HHS/United States
- R01 GM024872/GM/NIGMS NIH HHS/United States
- R01 MH059566/MH/NIMH NIH HHS/United States
- R01 GM24872/GM/NIGMS NIH HHS/United States
- R01 MH060879/MH/NIMH NIH HHS/United States
- R01 MH059571/MH/NIMH NIH HHS/United States
- R01 MH059565/MH/NIMH NIH HHS/United States
- T32 GM007863/GM/NIGMS NIH HHS/United States
- R01 MH059587/MH/NIMH NIH HHS/United States
- T32 GM07863/GM/NIGMS NIH HHS/United States
- R01 MH063420/MH/NIMH NIH HHS/United States
- U24 AG21886/AG/NIA NIH HHS/United States
- R01 MH059588/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

