Potential prostate cancer drug target: bioactivation of androstanediol by conversion to dihydrotestosterone
- PMID: 21705451
- PMCID: PMC3177006
- DOI: 10.1158/1078-0432.CCR-11-0644
Potential prostate cancer drug target: bioactivation of androstanediol by conversion to dihydrotestosterone
Abstract
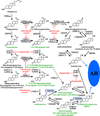
High-affinity binding of dihydrotestosterone (DHT) to the androgen receptor (AR) initiates androgen-dependent gene activation, required for normal male sex development in utero, and contributes to prostate cancer development and progression in men. Under normal physiologic conditions, DHT is synthesized predominantly by 5α-reduction of testosterone, the major circulating androgen produced by the testis. During androgen deprivation therapy, intratumoral androgen production is sufficient for AR activation and prostate cancer growth, even though circulating testicular androgen levels are low. Recent studies indicate that the metabolism of 5α-androstane-3α, 17β-diol by 17β-hydroxysteroid dehydrogenase 6 in benign prostate and prostate cancer cells is a major biosynthetic pathway for intratumoral synthesis of DHT, which binds AR and initiates transactivation to promote prostate cancer growth during androgen deprivation therapy. Drugs that target the so-called backdoor pathway of DHT synthesis provide an opportunity to enhance clinical response to luteinizing-hormone-releasing hormone (LHRH) agonists or antagonists, AR antagonists, and inhibitors of 5α-reductase enzymes (finasteride or dutasteride), and other steroid metabolism enzyme inhibitors (ketoconazole or the recently available abiraterone acetate).
©2011 AACR.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Figures
References
-
- Wilson EM, French FS. Binding properties of androgen receptors: evidence for identical receptors in rat testis, epididymis, and prostate. J Biol Chem. 1976;251:5620–5629. - PubMed
-
- Kemppainen JA, Langley E, Wong CI, Bobseine K, Kelce WR, Wilson EM. Distinguishing androgen receptor agonists and antagonists: distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone. Mol Endocrinol. 1999;13:440–454. - PubMed
-
- He B, Kemppainen JA, Wilson EM. FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J Biol Chem. 2000;275:22986–22994. - PubMed
-
- Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126:1165–1172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

