Colocalization and regulated physical association of presynaptic serotonin transporters with A₃ adenosine receptors
- PMID: 21705486
- PMCID: PMC3164334
- DOI: 10.1124/mol.111.071399
Colocalization and regulated physical association of presynaptic serotonin transporters with A₃ adenosine receptors
Abstract
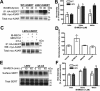
Activation of A₃ adenosine receptors (A₃ARs) rapidly enhances the activity of antidepressant-sensitive serotonin (5-HT) transporters (SERTs) in vitro, ex vivo, and in vivo. A₃AR agonist stimulation of SERT activity is lost in A₃AR knockout mice. A₃AR-stimulated SERT activity is mediated by protein kinase G1 (PKGI)- and p38 mitogen-activated protein kinase (MAPK)-linked pathways that support, respectively, enhanced SERT surface expression and catalytic activation. The mechanisms by which A₃ARs target SERTs among other potential effectors is unknown. Here we present evidence that A₃ARs are coexpressed with SERT in midbrain serotonergic neurons and form a physical complex in A₃AR/hSERT cotransfected cells. Treatment of A₃AR/SERT-cotransfected Chinese hamster ovary cells with the A₃AR agonist N⁶-(3-iodobenzyl)-N-methyl-5'-carbamoyladenosine (1 μM, 10 min), conditions previously reported to increase SERT surface expression and 5-HT uptake activity, enhanced the abundance of A₃AR/SERT complexes in a PKGI-dependent manner. Cotransfection of SERT with L90V-A₃AR, a hyperfunctional coding variant identified in subjects with autism spectrum disorder, resulted in a prolonged recovery of receptor/transporter complexes after A₃AR activation. Because PKGI and nitric-oxide synthetase are required for A₃AR stimulation of SERT activity, and proteins PKGI and NOS both form complexes with SERT, our findings suggest a mechanism by which signaling pathways coordinating A₃AR signaling to SERT can be spatially restricted and regulated, as well as compromised by neuropsychiatric disorders.
Figures





References
-
- Ansah TA, Ramamoorthy S, Montañez S, Daws LC, Blakely RD. (2003) Calcium-dependent inhibition of synaptosomal serotonin transport by the α2-adrenoceptor agonist 5-bromo-N-[4,5-dihydro-1H-imidazol-2-yl]-6-quinoxalinamine (UK14304). J Pharmacol Exp Ther 305:956–965 - PubMed
-
- Blakely RD, Defelice LJ, Galli A. (2005) Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology (Bethesda) 20:225–231 - PubMed
-
- Blakely RD, Ramamoorthy S, Schroeter S, Qian Y, Apparsundaram S, Galli A, DeFelice LJ. (1998) Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol Psychiatry 44:169–178 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA007390/DA/NIDA NIH HHS/United States
- R01 NS049261/NS/NINDS NIH HHS/United States
- NS049261/NS/NINDS NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- UL1-RR024975/RR/NCRR NIH HHS/United States
- R01 MH094527/MH/NIMH NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- R01-MH078028/MH/NIMH NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P50 MH078028/MH/NIMH NIH HHS/United States
- R01-MH94527/MH/NIMH NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- DK2093/DK/NIDDK NIH HHS/United States
- R01-DA07390/DA/NIDA NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

