Overexpression of Ste20-related proline/alanine-rich kinase exacerbates experimental colitis in mice
- PMID: 21705622
- PMCID: PMC3140558
- DOI: 10.4049/jimmunol.1002910
Overexpression of Ste20-related proline/alanine-rich kinase exacerbates experimental colitis in mice
Abstract
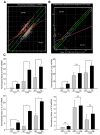
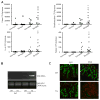
Inflammatory bowel disease, mainly Crohn's disease and ulcerative colitis, are characterized by epithelial barrier disruption and altered immune regulation. Colonic Ste20-like proline/alanine-rich kinase (SPAK) plays a role in intestinal inflammation, but its underlying mechanisms need to be defined. Both SPAK-transfected Caco2-BBE cells and villin-SPAK transgenic (TG) FVB/6 mice exhibited loss of intestinal barrier function. Further studies demonstrated that SPAK significantly increased paracellular intestinal permeability to FITC-dextran. In vivo studies using the mouse models of colitis induced by dextran sulfate sodium (DSS) and trinitrobenzene sulfonic acid showed that TG FVB/6 mice were more susceptible to DSS and trinitrobenzene sulfonic acid treatment than wild-type FVB/6 mice, as demonstrated by clinical and histological characteristics and enzymatic activities. Consistent with this notion, we found that SPAK increased intestinal epithelial permeability, which likely facilitated the production of inflammatory cytokines in vitro and in vivo, aggravated bacterial translocation in TG mice under DSS treatment, and consequently established a context favorable for the triggering of intestinal inflammation cascades. In conclusion, overexpression of SPAK inhibits maintenance of intestinal mucosal innate immune homeostasis, which makes regulation of SPAK important to attenuate pathological responses in inflammatory bowel disease.
Figures





Similar articles
-
Knockout of Ste20-like proline/alanine-rich kinase (SPAK) attenuates intestinal inflammation in mice.Am J Pathol. 2013 May;182(5):1617-28. doi: 10.1016/j.ajpath.2013.01.028. Epub 2013 Mar 13. Am J Pathol. 2013. PMID: 23499375
-
Cloning and characterization of a new intestinal inflammation-associated colonic epithelial Ste20-related protein kinase isoform.Biochim Biophys Acta. 2007 Feb;1769(2):106-16. doi: 10.1016/j.bbaexp.2007.01.003. Epub 2007 Jan 23. Biochim Biophys Acta. 2007. PMID: 17321610 Free PMC article.
-
Ste20-related proline/alanine-rich kinase (SPAK) regulated transcriptionally by hyperosmolarity is involved in intestinal barrier function.PLoS One. 2009;4(4):e5049. doi: 10.1371/journal.pone.0005049. Epub 2009 Apr 3. PLoS One. 2009. PMID: 19343169 Free PMC article.
-
Ste20-related proline/alanine-rich kinase: a novel regulator of intestinal inflammation.World J Gastroenterol. 2008 Oct 28;14(40):6115-21. doi: 10.3748/wjg.14.6115. World J Gastroenterol. 2008. PMID: 18985800 Free PMC article. Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Modulation of Cerebrospinal Fluid Dysregulation via a SPAK and OSR1 Targeted Framework Nucleic Acid in Hydrocephalus.Adv Sci (Weinh). 2024 May;11(17):e2306622. doi: 10.1002/advs.202306622. Epub 2024 Feb 14. Adv Sci (Weinh). 2024. PMID: 38353402 Free PMC article.
-
SPAK Deficiency Attenuates Chemotherapy-Induced Intestinal Mucositis.Front Oncol. 2021 Nov 23;11:733555. doi: 10.3389/fonc.2021.733555. eCollection 2021. Front Oncol. 2021. PMID: 34888232 Free PMC article.
-
Pathways to Parkinsonism Redux: convergent pathobiological mechanisms in genetics of Parkinson's disease.Hum Mol Genet. 2015 Oct 15;24(R1):R32-44. doi: 10.1093/hmg/ddv236. Epub 2015 Jun 22. Hum Mol Genet. 2015. PMID: 26101198 Free PMC article. Review.
-
Loss of TMF/ARA160 protein renders colonic mucus refractory to bacterial colonization and diminishes intestinal susceptibility to acute colitis.J Biol Chem. 2012 Jul 20;287(30):25631-9. doi: 10.1074/jbc.M112.364786. Epub 2012 May 2. J Biol Chem. 2012. PMID: 22553199 Free PMC article.
-
Inflammatory hydrocephalus.Childs Nerv Syst. 2021 Nov;37(11):3341-3353. doi: 10.1007/s00381-021-05255-z. Epub 2021 Jun 23. Childs Nerv Syst. 2021. PMID: 34164718
References
-
- Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. - PubMed
-
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. - PubMed
-
- Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

