Unique translation initiation of mRNAs-containing TISU element
- PMID: 21705780
- PMCID: PMC3177215
- DOI: 10.1093/nar/gkr484
Unique translation initiation of mRNAs-containing TISU element
Abstract
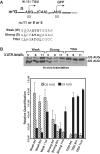
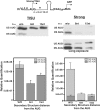
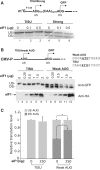
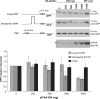
Translation Initiator of Short 5' UTR (TISU) is a unique regulatory element of both transcription and translation initiation. It is present in a sizable number of genes with basic cellular functions and a very short untranslated region (5' UTR). Here, we investigated translation initiation from short 5' UTR mRNAs with AUG in various contexts. Reducing 5' UTR length to the minimal functional size increases leaky scanning from weak and strong initiators but hardly affects translation initiation and ribosomal binding directed by TISU. Ribosome interaction with TISU mRNA is cap dependent and involves AUG downstream nucleotides that compensate for the absent 5' UTR contacts. Interestingly, eIF1 inhibits cap-proximal AUG selection within weak or strong contexts but not within TISU. Furthermore, TISU-directed translation is unaffected by inhibition of the RNA helicase eIF4A. Thus, TISU directs efficient cap-dependent translation initiation without scanning, a mechanism that would be advantageous when intracellular levels of eIF1 and eIF4A fluctuate.
Figures



References
-
- Marintchev A, Wagner G. Translation initiation: structures, mechanisms and evolution. Q. Rev. Biophys. 2004;37:197–284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

