RUNX3 acts as a tumor suppressor in breast cancer by targeting estrogen receptor α
- PMID: 21706051
- PMCID: PMC3697905
- DOI: 10.1038/onc.2011.252
RUNX3 acts as a tumor suppressor in breast cancer by targeting estrogen receptor α
Abstract
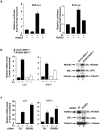
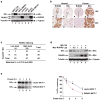
Transcription factor RUNX3 is inactivated in a number of malignancies, including breast cancer, and is suggested to function as a tumor suppressor. How RUNX3 functions as a tumor suppressor in breast cancer remains undefined. Here, we show that about 20% of female Runx3(+/-) mice spontaneously developed ductal carcinoma at an average age of 14.5 months. Additionally, RUNX3 inhibits the estrogen-dependent proliferation and transformation potential of ERα-positive MCF-7 breast cancer cells in liquid culture and in soft agar and suppresses the tumorigenicity of MCF-7 cells in severe combined immunodeficiency mice. Furthermore, RUNX3 inhibits ERα-dependent transactivation by reducing the stability of ERα. Consistent with its ability to regulate the levels of ERα, expression of RUNX3 inversely correlates with the expression of ERα in breast cancer cell lines, human breast cancer tissues and Runx3(+/-) mouse mammary tumors. By destabilizing ERα, RUNX3 acts as a novel tumor suppressor in breast cancer.
Conflict of interest statement
Figures


References
-
- Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–7. - PubMed
-
- Duong V, Boulle N, Daujat S, Chauvet J, Bonnet S, Neel H, et al. Differential regulation of estrogen receptor alpha turnover and transactivation by Mdm2 and stress-inducing agents. Cancer Res. 2007;67:5513–21. - PubMed
-
- Ekena K, Weis KE, Katzenellenbogen JA, Katzenellenbogen BS. Identification of amino acids in the hormone binding domain of the human estrogen receptor important in estrogen binding. J Biol Chem. 1996;271:20053–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

