Copper redox cycling in the prion protein depends critically on binding mode
- PMID: 21707094
- PMCID: PMC3166251
- DOI: 10.1021/ja2045259
Copper redox cycling in the prion protein depends critically on binding mode
Abstract
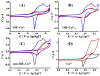
The prion protein (PrP) takes up 4-6 equiv of copper in its extended N-terminal domain, composed of the octarepeat (OR) segment (human sequence residues 60-91) and two mononuclear binding sites (at His96 and His111; also referred to as the non-OR region). The OR segment responds to specific copper concentrations by transitioning from a multi-His mode at low copper levels to a single-His, amide nitrogen mode at high levels (Chattopadhyay et al. J. Am. Chem. Soc. 2005, 127, 12647-12656). The specific function of PrP in healthy tissue is unclear, but numerous reports link copper uptake to a neuroprotective role that regulates cellular stress (Stevens, et al. PLoS Pathog.2009, 5 (4), e1000390). A current working hypothesis is that the high occupancy binding mode quenches copper's inherent redox cycling, thus, protecting against the production of reactive oxygen species from unregulated Fenton type reactions. Here, we directly test this hypothesis by performing detailed pH-dependent electrochemical measurements on both low and high occupancy copper binding modes. In contrast to the current belief, we find that the low occupancy mode completely quenches redox cycling, but high occupancy leads to the gentle production of hydrogen peroxide through a catalytic reduction of oxygen facilitated by the complex. These electrochemical findings are supported by independent kinetic measurements that probe for ascorbate usage and also peroxide production. Hydrogen peroxide production is also observed from a segment corresponding to the non-OR region. Collectively, these results overturn the current working hypothesis and suggest, instead, that the redox cycling of copper bound to PrP in the high occupancy mode is not quenched, but is regulated. The observed production of hydrogen peroxide suggests a mechanism that could explain PrP's putative role in cellular signaling.
© 2011 American Chemical Society
Figures
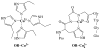




Similar articles
-
Difference in redox behaviors between copper-binding octarepeat and nonoctarepeat sites in prion protein.J Biol Inorg Chem. 2009 Nov;14(8):1209-18. doi: 10.1007/s00775-009-0564-y. Epub 2009 Jul 8. J Biol Inorg Chem. 2009. PMID: 19585160
-
The non-octarepeat copper binding site of the prion protein is a key regulator of prion conversion.Sci Rep. 2015 Oct 20;5:15253. doi: 10.1038/srep15253. Sci Rep. 2015. PMID: 26482532 Free PMC article.
-
Prion protein does not redox-silence Cu2+, but is a sacrificial quencher of hydroxyl radicals.Free Radic Biol Med. 2007 Jan 1;42(1):79-89. doi: 10.1016/j.freeradbiomed.2006.09.019. Epub 2006 Sep 27. Free Radic Biol Med. 2007. PMID: 17157195
-
Copper binding extrinsic to the octarepeat region in the prion protein.Curr Protein Pept Sci. 2009 Oct;10(5):529-35. doi: 10.2174/138920309789352056. Curr Protein Pept Sci. 2009. PMID: 19538144 Free PMC article. Review.
-
Insights into prion protein function from atomistic simulations.Prion. 2010 Jan-Mar;4(1):13-9. doi: 10.4161/pri.4.1.10969. Epub 2010 Jan 16. Prion. 2010. PMID: 20118658 Free PMC article. Review.
Cited by
-
Structural Determinants of the Prion Protein N-Terminus and Its Adducts with Copper Ions.Int J Mol Sci. 2018 Dec 20;20(1):18. doi: 10.3390/ijms20010018. Int J Mol Sci. 2018. PMID: 30577569 Free PMC article. Review.
-
Prion protein with a mutant N-terminal octarepeat region undergoes cobalamin-dependent assembly into high-molecular weight complexes.J Biol Chem. 2022 Apr;298(4):101770. doi: 10.1016/j.jbc.2022.101770. Epub 2022 Mar 7. J Biol Chem. 2022. PMID: 35271850 Free PMC article.
-
Gold nanoparticle-based colorimetric method for the detection of prostate-specific antigen.Int J Nanomedicine. 2018 Apr 24;13:2521-2530. doi: 10.2147/IJN.S154046. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29731627 Free PMC article.
-
Evolutionary implications of metal binding features in different species' prion protein: an inorganic point of view.Biomolecules. 2014 May 23;4(2):546-65. doi: 10.3390/biom4020546. Biomolecules. 2014. PMID: 24970230 Free PMC article. Review.
-
Prion protein and aging.Front Cell Dev Biol. 2014 Aug 29;2:44. doi: 10.3389/fcell.2014.00044. eCollection 2014. Front Cell Dev Biol. 2014. PMID: 25364751 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

