Role for Sit4p-dependent mitochondrial dysfunction in mediating the shortened chronological lifespan and oxidative stress sensitivity of Isc1p-deficient cells
- PMID: 21707788
- PMCID: PMC3133821
- DOI: 10.1111/j.1365-2958.2011.07714.x
Role for Sit4p-dependent mitochondrial dysfunction in mediating the shortened chronological lifespan and oxidative stress sensitivity of Isc1p-deficient cells
Abstract
Saccharomyces cerevisiae cells lacking Isc1p, an orthologue of mammalian neutral sphingomyelinase 2, display a shortened lifespan and an increased sensitivity to oxidative stress. A lipidomic analysis revealed specific changes in sphingolipids that accompanied the premature ageing of Isc1p-deficient cells under severe calorie restriction conditions, including a decrease of dihydrosphingosine levels and an increase of dihydro-C(26) -ceramide and phyto-C(26) -ceramide levels, the latter raising the possibility of activation of ceramide-dependent protein phosphatases. Consequently, deletion of the SIT4 gene, which encodes for the catalytic subunit of type 2A ceramide-activated protein phosphatase in yeast, abolished the premature ageing and hydrogen peroxide sensitivity of isc1Δ cells. SIT4 deletion also abolished the respiratory defects and catalase A deficiency exhibited by isc1Δ mutants. These results are consistent with catabolic derepression associated with the loss of Sit4p. The overall results show that Isc1p is an upstream regulator of Sit4p and implicate Sit4p activation in mitochondrial dysfunction leading to the shortened chronological lifespan and oxidative stress sensitivity of isc1Δ mutants.
© 2011 Blackwell Publishing Ltd.
Figures
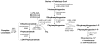






















References
-
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. - PubMed
-
- Aerts AM, Francois IE, Bammens L, Cammue BP, Smets B, Winderickx J, Accardo S, De Vos DE, Thevissen K. Level of M(IP)2C sphingolipid affects plant defensin sensitivity, oxidative stress resistance and chronological life-span in yeast. FEBS Lett. 2006;580:1903–1907. - PubMed
-
- Almeida T, Marques M, Mojzita D, Amorim MA, Silva RD, Almeida B, Rodrigues P, Ludovico P, Hohmann S, Moradas-Ferreira P, Corte-Real M, Costa V. Isc1p plays a key role in hydrogen peroxide resistance and chronological lifespan through modulation of iron levels and apoptosis. Mol Biol Cell. 2008;19:865–876. - PMC - PubMed
-
- Angeles de la Torre-Ruiz M, Torres J, Arino J, Herrero E. Sit4 is required for proper modulation of the biological functions mediated by Pkc1 and the cell integrity pathway in Saccharomyces cerevisiae. J Biol Chem. 2002;277:33468–33476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

