Enhanced mobilization of the bone marrow-derived circulating progenitor cells by intracoronary freshly isolated bone marrow cells transplantation in patients with acute myocardial infarction
- PMID: 21707914
- PMCID: PMC3822854
- DOI: 10.1111/j.1582-4934.2011.01358.x
Enhanced mobilization of the bone marrow-derived circulating progenitor cells by intracoronary freshly isolated bone marrow cells transplantation in patients with acute myocardial infarction
Abstract
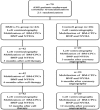
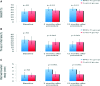
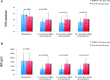
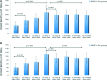
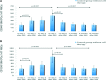
Autologous bone marrow cell transplantation (BMCs-Tx) is a promising novel option for treatment of cardiovascular disease. We analysed in a randomized controlled study the influence of the intracoronary autologous freshly isolated BMCs-Tx on the mobilization of bone marrow-derived circulating progenitor cells (BM-CPCs) in patients with acute myocardial infarction (AMI). Sixty-two patients with AMI were randomized to either freshly isolated BMCs-Tx or to a control group without cell therapy. Peripheral blood (PB) concentrations of CD34/45(+) - and CD133/45(+)-circulating progenitor cells were measured by flow cytometry in 42 AMI patients with cell therapy as well as in 20 AMI patients without cell therapy as a control group on days 1, 3, 5, 7, 8 and 3, 6 as well as 12 months after AMI. Global ejection fraction (EF) and the size of infarct area were determined by left ventriculography. We observed in patients with freshly isolated BMCs-Tx at 3 and 12 months follow up a significant reduction of infarct size and increase of global EF as well as infarct wall movement velocity. The mobilization of CD34/45(+) and CD133/45(+) BM-CPCs significantly increased with a peak on day 7 as compared to baseline after AMI in both groups (CD34/45(+): P < 0.001, CD133/45(+): P < 0.001). Moreover, this significant mobilization of BM-CPCs existed 3, 6 and 12 months after cell therapy compared to day 1 after AMI. In control group, there were no significant differences of CD34/45(+) and CD133/45(+) BM-CPCs mobilization between day 1 and 3, 6 and 12 months after AMI. Intracoronary transplantation of autologous freshly isolated BMCs by use of point of care system in patients with AMI may enhance and prolong the mobilization of CD34/45(+) and CD133/45(+) BM-CPCs in PB and this might increase the regenerative potency after AMI.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

References
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. - PubMed
-
- Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. - PubMed
-
- Gunsilius E, Duba HC, Petzer A, et al. Contribution of endothelial cells of hematopoietic origin to blood vessel formation. Circ Res. 2001;88:E1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

