Involvement of gut microbial fermentation in the metabolic alterations occurring in n-3 polyunsaturated fatty acids-depleted mice
- PMID: 21707971
- PMCID: PMC3141392
- DOI: 10.1186/1743-7075-8-44
Involvement of gut microbial fermentation in the metabolic alterations occurring in n-3 polyunsaturated fatty acids-depleted mice
Abstract
Background: Western diet is characterized by an insufficient n-3 polyunsaturated fatty acid (PUFA) consumption which is known to promote the pathogenesis of several diseases. We have previously observed that mice fed with a diet poor in n-3 PUFA for two generations exhibit hepatic steatosis together with a decrease in body weight. The gut microbiota contributes to the regulation of host energy metabolism, due to symbiotic relationship with fermentable nutrients provided in the diet. In this study, we have tested the hypothesis that perturbations of the gut microbiota contribute to the metabolic alterations occurring in mice fed a diet poor in n-3 PUFA for two generations (n-3/- mice).
Methods: C57Bl/6J mice fed with a control or an n-3 PUFA depleted diet for two generations were supplemented with prebiotic (inulin-type Fructooligosaccharides, FOS, 0.20 g/day/mice) during 24 days.
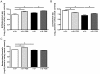
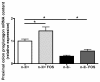
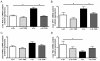
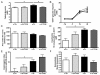
Results: n-3/-mice exhibited a marked drop in caecum weight, a decrease in lactobacilli and an increase in bifidobacteria in the caecal content as compared to control mice (n-3/+ mice). Dietary supplementation with FOS for 24 days was sufficient to increase caecal weight and bifidobacteria count in both n-3/+ and n-3/-mice. Moreover, FOS increased lactobacilli content in n-3/-mice, whereas it decreased their level in n-3/+ mice. Interestingly, FOS treatment promoted body weight gain in n-3/-mice by increasing energy efficiency. In addition, FOS treatment decreased fasting glycemia and lowered the higher expression of key factors involved in the fatty acid catabolism observed in the liver of n-3/-mice, without lessening steatosis.
Conclusions: the changes in the gut microbiota composition induced by FOS are different depending on the type of diet. We show that FOS may promote lactobacilli and counteract the catabolic status induced by n-3 PUFA depletion in mice, thereby contributing to restore efficient fat storage.
Figures
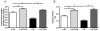
References
-
- Browning LM, Krebs JD, Moore CS, Mishra GD, O'Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab. 2007;9:70–80. doi: 10.1111/j.1463-1326.2006.00576.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

