Defective response of CD4(+) T cells to retinoic acid and TGFβ in systemic lupus erythematosus
- PMID: 21708033
- PMCID: PMC3218921
- DOI: 10.1186/ar3387
Defective response of CD4(+) T cells to retinoic acid and TGFβ in systemic lupus erythematosus
Abstract
Introduction: CD25(+) FOXP3(+) CD4(+) regulatory T cells (Tregs) are induced by transforming growth factor β (TGFβ) and further expanded by retinoic acid (RA). We have previously shown that this process was defective in T cells from lupus-prone mice expressing the novel isoform of the Pbx1 gene, Pbx1-d. This study tested the hypothesis that CD4(+) T cells from systemic lupus erythematosus (SLE) patients exhibited similar defects in Treg induction in response to TGFβ and RA, and that PBX1-d expression is associated with this defect.
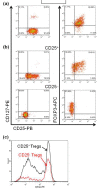
Methods: Peripheral blood mononuclear cells (PBMCs) were collected from 142 SLE patients and 83 healthy controls (HCs). The frequency of total, memory and naïve CD4(+) T cells was measured by flow cytometry on fresh cells. PBX1 isoform expression in purified CD4(+) T cells was determined by reverse transcription polymerase chain reaction (RT-PCR). PBMCs were stimulated for three days with anti-CD3 and anti-CD28 in the presence or absence of TGFβ and RA. The expression of CD25 and FOXP3 on CD4(+) T cells was then determined by flow cytometry. In vitro suppression assays were performed with sorted CD25(+) and CD25(-) FOXP3(+) T cells. CD4(+) T cell subsets or their expansion were compared between patients and HCs with two-tailed Mann-Whitney tests and correlations between the frequencies of two subsets were tested with Spearman tests.
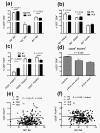
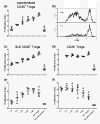
Results: The percentage of CD25(-) FOXP3(+) CD4(+) (CD25(-) Tregs) T cells was greater in SLE patients than in HCs, but these cells, contrary to their matched CD25(+) counterparts, did not show a suppressive activity. RA-expansion of TGFβ-induced CD25(+) Tregs was significantly lower in SLE patients than in HCs, although SLE Tregs expanded significantly more than HCs in response to either RA or TGFβ alone. Defective responses were also observed for the SLE CD25(-) Tregs and CD25(+) FOXP3(-) activated CD4(+) T cells as compared to controls. PBX1-d expression did not affect Treg induction, but it significantly reduced the expansion of CD25- Tregs and prevented the reduction of the activated CD25(+) FOXP3(-) CD4(+) T cell subset by the combination of TGFβ and RA.
Conclusions: We demonstrated that the induction of Tregs by TGFβ and RA was defective in SLE patients and that PBX1-d expression in CD4(+) T cells is associated with an impaired regulation of FOXP3 and CD25 by TGFβ and RA on these cells. These results suggest an impaired integration of the TGFβ and RA signals in SLE T cells and implicate the PBX1 gene in this process.
Figures


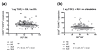
Similar articles
-
Low expressions of PD-L1 and CTLA-4 by induced CD4+CD25+ Foxp3+ Tregs in patients with SLE and their correlation with the disease activity.Cytokine. 2020 Sep;133:155119. doi: 10.1016/j.cyto.2020.155119. Epub 2020 Jun 11. Cytokine. 2020. PMID: 32535334
-
CD4(+)CD25(+)CD127(-) and CD4(+)CD25(+)Foxp3(+) Regulatory T Cell Subsets in Mediating Autoimmune Reactivity in Systemic Lupus Erythematosus Patients.Arch Immunol Ther Exp (Warsz). 2016 Oct;64(5):399-407. doi: 10.1007/s00005-016-0399-5. Epub 2016 May 7. Arch Immunol Ther Exp (Warsz). 2016. PMID: 27156107 Free PMC article.
-
CD4+CD69+ T cells and CD4+CD25+FoxP3+ Treg cells imbalance in peripheral blood, spleen and peritoneal lavage from pristane-induced systemic lupus erythematosus (SLE) mice.Adv Rheumatol. 2019 Jul 24;59(1):30. doi: 10.1186/s42358-019-0072-x. Adv Rheumatol. 2019. PMID: 31340848
-
The Proportion of Regulatory T Cells in Patients with Systemic Lupus Erythematosus: A Meta-Analysis.J Immunol Res. 2018 Sep 3;2018:7103219. doi: 10.1155/2018/7103219. eCollection 2018. J Immunol Res. 2018. PMID: 30255107 Free PMC article. Review.
-
Identity of mysterious CD4+CD25-Foxp3+ cells in SLE.Arthritis Res Ther. 2010;12(1):101. doi: 10.1186/ar2894. Epub 2010 Jan 20. Arthritis Res Ther. 2010. PMID: 20122288 Free PMC article. Review.
Cited by
-
Metabolic Factors that Contribute to Lupus Pathogenesis.Crit Rev Immunol. 2016;36(1):75-98. doi: 10.1615/CritRevImmunol.2016017164. Crit Rev Immunol. 2016. PMID: 27480903 Free PMC article. Review.
-
The peroxisome-proliferator activated receptor-γ agonist pioglitazone modulates aberrant T cell responses in systemic lupus erythematosus.Clin Immunol. 2013 Oct;149(1):119-32. doi: 10.1016/j.clim.2013.07.002. Epub 2013 Jul 20. Clin Immunol. 2013. PMID: 23962407 Free PMC article.
-
Paradoxical effects of all-trans-retinoic acid on lupus-like disease in the MRL/lpr mouse model.PLoS One. 2015 Mar 16;10(3):e0118176. doi: 10.1371/journal.pone.0118176. eCollection 2015. PLoS One. 2015. PMID: 25775135 Free PMC article.
-
Tolerogenic dendritic cells generated with dexamethasone and vitamin D3 regulate rheumatoid arthritis CD4+ T cells partly via transforming growth factor-β1.Clin Exp Immunol. 2017 Jan;187(1):113-123. doi: 10.1111/cei.12870. Epub 2016 Nov 2. Clin Exp Immunol. 2017. PMID: 27667787 Free PMC article.
-
The SLE-associated Pbx1-d isoform acts as a dominant-negative transcriptional regulator.Genes Immun. 2012 Dec;13(8):653-7. doi: 10.1038/gene.2012.43. Epub 2012 Sep 20. Genes Immun. 2012. PMID: 22992721 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

