Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells
- PMID: 21708104
- PMCID: PMC3494970
- DOI: 10.1053/j.gastro.2011.06.049
Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells
Abstract
BACKGROUND& AIMS: Embryonic biliary precursor cells form a periportal sheet called the ductal plate, which is progressively remodeled to generate intrahepatic bile ducts. A limited number of ductal plate cells participate in duct formation; those not involved in duct development are believed to involute by apoptosis. Moreover, cells that express the SRY-related HMG box transcription factor 9 (SOX9), which include the embryonic ductal plate cells, were proposed to continuously supply the liver with hepatic cells. We investigated the role of the ductal plate in hepatic morphogenesis.
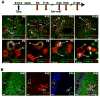
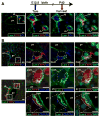
Methods: Apoptosis and proliferation were investigated by immunostaining of mouse and human fetal liver tissue. The postnatal progeny of SOX9-expressing ductal plate cells was analyzed after genetic labeling, at the ductal plate stage, by Cre-mediated recombination of a ROSA26RYFP reporter allele. Inducible Cre expression was induced by SOX9 regulatory regions, inserted in a bacterial artificial chromosome. Livers were studied from mice under normal conditions and during diet-induced regeneration.
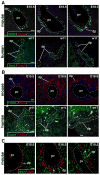
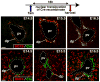
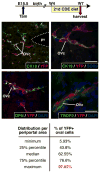
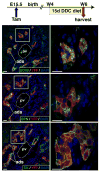
Results: Ductal plate cells did not undergo apoptosis and showed limited proliferation. They generated cholangiocytes lining interlobular bile ducts, bile ductules, and canals of Hering, as well as periportal hepatocytes. Oval cells that appeared during regeneration also derived from the ductal plate. We did not find that liver homeostasis required a continuous supply of cells from SOX9-expressing progenitors.
Conclusions: The ductal plate gives rise to cholangiocytes lining the intrahepatic bile ducts, including its most proximal segments. It also generates periportal hepatocytes and adult hepatic progenitor cells.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures: The authors declare no conflict of interest.
Figures
Comment in
-
The ductal plate: a source of progenitors and hepatocytes in the adult liver.Gastroenterology. 2011 Oct;141(4):1152-5. doi: 10.1053/j.gastro.2011.08.023. Epub 2011 Aug 27. Gastroenterology. 2011. PMID: 21875588 No abstract available.
-
Differentiation of intrahepatic peribiliary glands and pancreatic acinar cells from the remodeling ductal plate in human fetuses.Hepatology. 2012 Nov;56(5):2004-5. doi: 10.1002/hep.25750. Hepatology. 2012. PMID: 22473877 No abstract available.
References
-
- Jochheim A, Cieslak A, Hillemann T, et al. Multi-stage analysis of differential gene expression in BALB/C mouse liver development by high-density microarrays. Differentiation. 2003;71:62–72. - PubMed
-
- Petkov PM, Zavadil J, Goetz D, et al. Gene expression pattern in hepatic stem/progenitor cells during rat fetal development using complementary DNA microarrays. Hepatology. 2004;39:617–27. - PubMed
-
- Torre C, Perret C, Colnot S. Molecular determinants of liver zonation. Prog Mol Biol Transl Sci. 2010;97:127–50. - PubMed
-
- Raynaud P, Carpentier R, Antoniou A, et al. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Biol. 2011;43:245–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

