Evolution of nubbin function in hemimetabolous and holometabolous insect appendages
- PMID: 21708143
- PMCID: PMC3178182
- DOI: 10.1016/j.ydbio.2011.06.014
Evolution of nubbin function in hemimetabolous and holometabolous insect appendages
Abstract
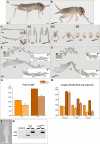
Insects display a whole spectrum of morphological diversity, which is especially noticeable in the organization of their appendages. A recent study in a hemipteran, Oncopeltus fasciatus (milkweed bug), showed that nubbin (nub) affects antenna morphogenesis, labial patterning, the length of the femoral segment in legs, and the formation of a limbless abdomen. To further determine the role of this gene in the evolution of insect morphology, we analyzed its functions in two additional hemimetabolous species, Acheta domesticus (house cricket) and Periplaneta americana (cockroach), and re-examined its role in Drosophila melanogaster (fruit fly). While both Acheta and Periplaneta nub-RNAi first nymphs develop crooked antennae, no visible changes are observed in the morphologies of their mouthparts and abdomen. Instead, the main effect is seen in legs. The joint between the tibia and first tarsomere (Ta-1) is lost in Acheta, which in turn, causes a fusion of these two segments and creates a chimeric nub-RNAi tibia-tarsus that retains a tibial identity in its proximal half and acquires a Ta-1 identity in its distal half. Similarly, our re-analysis of nub function in Drosophila reveals that legs lack all true joints and the fly tibia also exhibits a fused tibia and tarsus. Finally, we observe a similar phenotype in Periplaneta except that it encompasses different joints (coxa-trochanter and femur-tibia), and in this species we also show that nub expression in the legs is regulated by Notch signaling, as had previously been reported in flies and spiders. Overall, we propose that nub acts downstream of Notch on the distal part of insect leg segments to promote their development and growth, which in turn is required for joint formation. Our data represent the first functional evidence defining a role for nub in leg segmentation and highlight the varying degrees of its involvement in this process across insects.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Analysis of nubbin expression patterns in insects.Evol Dev. 2004 Sep-Oct;6(5):310-24. doi: 10.1111/j.1525-142X.2004.04039.x. Evol Dev. 2004. PMID: 15330864
-
RNAi analysis of nubbin embryonic functions in a hemimetabolous insect, Oncopeltus fasciatus.Evol Dev. 2008 Nov-Dec;10(6):705-16. doi: 10.1111/j.1525-142X.2008.00284.x. Evol Dev. 2008. PMID: 19021741
-
Functional analyses in the hemipteran Oncopeltus fasciatus reveal conserved and derived aspects of appendage patterning in insects.Dev Biol. 2004 Jul 15;271(2):306-21. doi: 10.1016/j.ydbio.2004.04.005. Dev Biol. 2004. PMID: 15223336
-
Evolution of insect development: to the hemimetabolous paradigm.Curr Opin Genet Dev. 2010 Aug;20(4):355-61. doi: 10.1016/j.gde.2010.04.005. Epub 2010 May 10. Curr Opin Genet Dev. 2010. PMID: 20462751 Review.
-
Hox3/zen and the evolution of extraembryonic epithelia in insects.Adv Exp Med Biol. 2010;689:133-44. doi: 10.1007/978-1-4419-6673-5_10. Adv Exp Med Biol. 2010. PMID: 20795328 Review.
Cited by
-
Gene regulatory dynamics during the development of a paleopteran insect, the mayfly Cloeon dipterum.Development. 2024 Oct 15;151(20):dev203017. doi: 10.1242/dev.203017. Epub 2024 Oct 10. Development. 2024. PMID: 39324209 Free PMC article.
-
Extent With Modification: Leg Patterning in the Beetle Tribolium castaneum and the Evolution of Serial Homologs.G3 (Bethesda). 2012 Feb;2(2):235-48. doi: 10.1534/g3.111.001537. Epub 2012 Feb 1. G3 (Bethesda). 2012. PMID: 22384402 Free PMC article.
-
Developmental system drift in the patterning of the arthropod tarsus.bioRxiv [Preprint]. 2025 Jul 11:2025.07.08.663771. doi: 10.1101/2025.07.08.663771. bioRxiv. 2025. PMID: 40672340 Free PMC article. Preprint.
-
Notch Signaling in Insect Development: A Simple Pathway with Diverse Functions.Int J Mol Sci. 2023 Sep 13;24(18):14028. doi: 10.3390/ijms241814028. Int J Mol Sci. 2023. PMID: 37762331 Free PMC article. Review.
-
Tarsal-less peptides control Notch signalling through the Shavenbaby transcription factor.Dev Biol. 2011 Jul 15;355(2):183-93. doi: 10.1016/j.ydbio.2011.03.033. Epub 2011 Apr 17. Dev Biol. 2011. PMID: 21527259 Free PMC article.
References
-
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–30. - PubMed
-
- Abzhanov A, Kaufman TC. Homologs of Drosophila appendage genes in the patterning of arthropod limbs. Dev Biol. 2000;227:673–89. - PubMed
-
- Angelini DR, Kaufman TC. Functional analyses in the hemipteran Oncopeltus fasciatus reveal conserved and derived aspects of appendage patterning in insects. Dev Biol. 2004;271:306–21. - PubMed
-
- Averof M, Patel NH. Crustacean appendage evolution associated with changes in Hox gene expression. Nature. 1997;388:682–6. - PubMed
-
- Beermann A, Jay DG, Beeman RW, Hulskamp M, Tautz D, Jurgens G. The Short antennae gene of Tribolium is required for limb development and encodes the orthologue of the Drosophila Distal-less protein. Development. 2001;128:287–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

