An efficient method for the long-term and specific expression of exogenous cDNAs in cultured Purkinje neurons
- PMID: 21708190
- PMCID: PMC3407467
- DOI: 10.1016/j.jneumeth.2011.06.006
An efficient method for the long-term and specific expression of exogenous cDNAs in cultured Purkinje neurons
Abstract
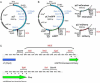
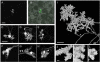
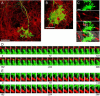
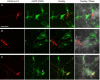
We present a simple and efficient method for expressing cDNAs in Purkinje neurons (PNs) present in heterogeneous mouse cerebellar cultures. The method combines the transfection of freshly dissociated cerebellar cells via nucleofection with the use of novel expression plasmids containing a fragment of the L7 (Pcp2) gene that, within the cerebellum, drives PN-specific expression. The efficiency of PN transfection (determined 13 days post nucleofection) is approximately 70%. Double and triple transfections are routinely achieved at slightly lower efficiencies. Expression in PNs is obvious after one week in culture and still strong after three weeks, by which time these neurons are well-developed. Moreover, high-level expression is restricted almost exclusively to the PNs present in these mixed cultures, which greatly facilitates the characterization of PN-specific functions. As proof of principle, we used this method to visualize (1) the morphology of living PNs expressing mGFP, (2) the localization and dynamics of the dendritic spine proteins PSD-93 and Homer-3a tagged with mGFP and (3) the interaction of live PNs expressing mGFP with other cerebellar neurons expressing mCherry from a β-Actin promoter plasmid. Finally, we created a series of L7-plasmids containing different fluorescent protein cDNAs that are suited for the expression of cDNAs of interest as N- and C-terminally tagged fluorescent fusion proteins. In summary, this procedure allows for the highly efficient, long-term, and specific expression of multiple cDNAs in differentiated PNs, and provides a favorable alternative to two procedures (viral transduction, ballistic gene delivery) used previously to express genes in cultured PNs.
Published by Elsevier B.V.
Figures


Similar articles
-
Optimization of cerebellar purkinje neuron cultures and development of a plasmid-based method for purkinje neuron-specific, miRNA-mediated protein knockdown.Methods Cell Biol. 2016;131:177-97. doi: 10.1016/bs.mcb.2015.06.004. Epub 2015 Sep 2. Methods Cell Biol. 2016. PMID: 26794514 Free PMC article.
-
An Improved Method for Differentiating Mouse Embryonic Stem Cells into Cerebellar Purkinje Neurons.Cerebellum. 2019 Jun;18(3):406-421. doi: 10.1007/s12311-019-1007-0. Cerebellum. 2019. PMID: 30729383 Free PMC article.
-
A promoter element with enhancer properties, and the orphan nuclear receptor RORalpha, are required for Purkinje cell-specific expression of a Gi/o modulator.Mol Cell Neurosci. 2007 Mar;34(3):324-42. doi: 10.1016/j.mcn.2006.11.013. Epub 2007 Jan 9. Mol Cell Neurosci. 2007. PMID: 17215137
-
Dendrite formation of cerebellar Purkinje cells.Neurochem Res. 2009 Dec;34(12):2078-88. doi: 10.1007/s11064-009-0073-y. Epub 2009 Oct 10. Neurochem Res. 2009. PMID: 19821027 Review.
-
Development and fine structure of murine Purkinje cells in dissociated cerebellar cultures: neuronal polarity.Anat Embryol (Berl). 1998 Jan;197(1):9-29. doi: 10.1007/s004290050117. Anat Embryol (Berl). 1998. PMID: 9462856 Review.
Cited by
-
Polycomb Ezh2 controls the fate of GABAergic neurons in the embryonic cerebellum.Development. 2016 Jun 1;143(11):1971-80. doi: 10.1242/dev.132902. Epub 2016 Apr 11. Development. 2016. PMID: 27068104 Free PMC article.
-
PKCγ-Mediated Phosphorylation of CRMP2 Regulates Dendritic Outgrowth in Cerebellar Purkinje Cells.Mol Neurobiol. 2020 Dec;57(12):5150-5166. doi: 10.1007/s12035-020-02038-6. Epub 2020 Aug 29. Mol Neurobiol. 2020. PMID: 32860158 Free PMC article.
-
Increased biological activity of protein Kinase C gamma is not required in Spinocerebellar ataxia 14.Mol Brain. 2017 Jul 24;10(1):34. doi: 10.1186/s13041-017-0313-z. Mol Brain. 2017. PMID: 28738819 Free PMC article.
-
Novel genetic features of human and mouse Purkinje cell differentiation defined by comparative transcriptomics.Proc Natl Acad Sci U S A. 2020 Jun 30;117(26):15085-15095. doi: 10.1073/pnas.2000102117. Epub 2020 Jun 16. Proc Natl Acad Sci U S A. 2020. PMID: 32546527 Free PMC article.
-
Optimization of cerebellar purkinje neuron cultures and development of a plasmid-based method for purkinje neuron-specific, miRNA-mediated protein knockdown.Methods Cell Biol. 2016;131:177-97. doi: 10.1016/bs.mcb.2015.06.004. Epub 2015 Sep 2. Methods Cell Biol. 2016. PMID: 26794514 Free PMC article.
References
-
- Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12:243–60. - PubMed
-
- Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–8. - PubMed
-
- Biewenga JE, Destree OH, Schrama LH. Plasmid-mediated gene transfer in neurons using the biolistics technique. J. Neurosci. Methods. 1997;71:67–75. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

