Relationship of electrophilic stress to aging
- PMID: 21708248
- PMCID: PMC3156362
- DOI: 10.1016/j.freeradbiomed.2011.05.039
Relationship of electrophilic stress to aging
Abstract
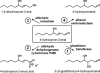
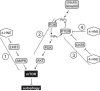
This review begins with the premise that an organism's life span is determined by the balance between two countervailing forces: (i) the sum of destabilizing effects and (ii) the sum of protective longevity-assurance processes. Against this backdrop, the role of electrophiles is discussed, both as destabilizing factors and as signals that induce protective responses. Because most biological macromolecules contain nucleophilic centers, electrophiles are particularly reactive and toxic in a biological context. The majority of cellular electrophiles are generated from polyunsaturated fatty acids by a peroxidation chain reaction that is readily triggered by oxygen-centered radicals, but propagates without further input of reactive oxygen species (ROS). Thus, the formation of lipid-derived electrophiles such as 4-hydroxynon-2-enal (4-HNE) is proposed to be relatively insensitive to the level of initiating ROS, but to depend mainly on the availability of peroxidation-susceptible fatty acids. This is consistent with numerous observations that life span is inversely correlated to membrane peroxidizability, and with the hypothesis that 4-HNE may constitute the mechanistic link between high susceptibility of membrane lipids to peroxidation and shortened life span. Experimental interventions that directly alter membrane composition (and thus their peroxidizability) or modulate 4-HNE levels have the expected effects on life span, establishing that the connection is not only correlative but causal. Specific molecular mechanisms are considered, by which 4-HNE could (i) destabilize biological systems via nontargeted reactions with cellular macromolecules and (ii) modulate signaling pathways that control longevity-assurance mechanisms.
Published by Elsevier Inc.
Figures


Similar articles
-
Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic beta cells.Mol Aspects Med. 2016 Jun;49:49-77. doi: 10.1016/j.mam.2016.03.001. Epub 2016 Mar 21. Mol Aspects Med. 2016. PMID: 27012748 Review.
-
Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal.Mech Ageing Dev. 2007 Feb;128(2):196-205. doi: 10.1016/j.mad.2006.11.025. Epub 2006 Dec 8. Mech Ageing Dev. 2007. PMID: 17157356 Free PMC article.
-
Electrophilic Aldehyde 4-Hydroxy-2-Nonenal Mediated Signaling and Mitochondrial Dysfunction.Biomolecules. 2022 Oct 25;12(11):1555. doi: 10.3390/biom12111555. Biomolecules. 2022. PMID: 36358905 Free PMC article. Review.
-
Tris(1,3-dichloro-2-propyl) phosphate accelerated the aging process induced by the 4-hydroxynon-2-enal response to reactive oxidative species in Caenorhabditis elegans.Environ Pollut. 2019 Mar;246:904-913. doi: 10.1016/j.envpol.2018.12.082. Epub 2018 Dec 28. Environ Pollut. 2019. PMID: 31159140
-
Antioxidants and HNE in redox homeostasis.Free Radic Biol Med. 2017 Oct;111:87-101. doi: 10.1016/j.freeradbiomed.2016.11.033. Epub 2016 Nov 22. Free Radic Biol Med. 2017. PMID: 27888001 Review.
Cited by
-
Regulatory roles of glutathione-S-transferases and 4-hydroxynonenal in stress-mediated signaling and toxicity.Free Radic Biol Med. 2017 Oct;111:235-243. doi: 10.1016/j.freeradbiomed.2016.10.493. Epub 2016 Oct 26. Free Radic Biol Med. 2017. PMID: 27794453 Free PMC article. Review.
-
What is the rate-limiting step towards aging? Chemical reaction kinetics might reconcile contradictory observations in experimental aging research.Geroscience. 2020 Jun;42(3):857-866. doi: 10.1007/s11357-019-00058-2. Epub 2019 Feb 27. Geroscience. 2020. PMID: 30809734 Free PMC article.
-
Reactive carbonyl species in vivo: generation and dual biological effects.ScientificWorldJournal. 2014 Jan 21;2014:417842. doi: 10.1155/2014/417842. eCollection 2014. ScientificWorldJournal. 2014. PMID: 24634611 Free PMC article. Review.
-
4-hydroxynonenal-mediated signaling and aging.Free Radic Biol Med. 2017 Oct;111:219-225. doi: 10.1016/j.freeradbiomed.2016.11.032. Epub 2016 Nov 20. Free Radic Biol Med. 2017. PMID: 27876535 Free PMC article. Review.
-
Protection from oxidative and electrophilic stress in the Gsta4-null mouse heart.Cardiovasc Toxicol. 2013 Dec;13(4):347-56. doi: 10.1007/s12012-013-9215-1. Cardiovasc Toxicol. 2013. PMID: 23690225 Free PMC article.
References
-
- Pinkston JM, Garigan D, Hansen M, Kenyon C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science. 2006;313:971–975. - PubMed
-
- Pinkston-Gosse J, Kenyon C. DAF-16/FOXO targets genes that regulate tumor growth in Caenorhabditis elegans. Nat. Genet. 2007;39:1403. - PubMed
-
- March J. Reactions, mechanisms, and structure. Wiley; New York: 1985. Advanced organic chemistry.
-
- Mayr H, Ofial AR. Kinetics of electrophile-nucleophile combinations: A general approach to polar organic reactivity. Pure Appl. Chem. 2005;77:1807–1821.
-
- Pearson RG. Hard and soft acids and bases - the evolution of a chemical concept. Coord. Chem. Rev. 1990;100:403–425.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

