The type II collagen N-propeptide, PIIBNP, inhibits cell survival and bone resorption of osteoclasts via integrin-mediated signaling
- PMID: 21708300
- PMCID: PMC3166963
- DOI: 10.1016/j.bone.2011.06.011
The type II collagen N-propeptide, PIIBNP, inhibits cell survival and bone resorption of osteoclasts via integrin-mediated signaling
Abstract
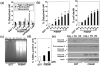
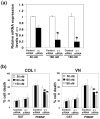
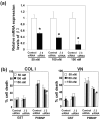
Objective: Type IIB procollagen is characteristic of cartilage, comprising 50% of the extracellular matrix. The NH(2)-propeptide of type IIB collagen, PIIBNP, can kill tumor cells via binding to integrins α(V)β(3) and α(V)β(5). As osteoclasts rely on α(V)β(3) integrins for function in bone erosion, we sought to determine whether PIIBNP could inhibit osteoclast function.
Methods: We undertook in vitro and in vivo experiments to evaluate both osteoblast and osteoclast functions in the presence of recombinant PIIBNP. Adhesion of osteoclasts to PIIBNP was analyzed by staining of attached cells with crystal violet. PIIBNP-induced cell death was evaluated by counting Trypan Blue stained cells. The mechanism of cell death was evaluated by DNA fragmentation, TUNEL staining and western blotting to detect cleaved caspases. To determine the role of α(V)β(3) integrin, osteoclasts were pretreated with α(V) or β(3) integrin specific siRNA before the treatment with PIIBNP. To explore PIIBNP function in vivo, a lipopolysaccharide-induced mouse calvaria lysis model was employed.
Results: Osteoclasts adhered to PIIBNP via an RGD-mediated mechanism. When osteoclasts were plated on extracellular matrix proteins, PIIBNP induced apoptosis of osteoclasts via caspase 3/8 activation. Osteoblasts and macrophages were not killed. Reduction of α(V) or β(3) integrin levels on osteoclasts by siRNA reduced cell death in a dose-dependent manner. In vivo, PIIBNP could inhibit bone resorption.
Conclusion: We conclude that PIIBNP can inhibit osteoclast survival and bone resorption via signal transduction through the α(V)β(3) integrins. Because of this property and the cell specificity, we propose that PIIBNP may play a role in vivo in protecting cartilage from osteoclast invasion and also could be a new therapeutic strategy for decreasing bone loss.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Type IIB procollagen NH(2)-propeptide induces death of tumor cells via interaction with integrins alpha(V)beta(3) and alpha(V)beta(5).J Biol Chem. 2010 Jul 2;285(27):20806-17. doi: 10.1074/jbc.M110.118521. Epub 2010 May 3. J Biol Chem. 2010. PMID: 20439458 Free PMC article.
-
Unoccupied alpha(v)beta3 integrin regulates osteoclast apoptosis by transmitting a positive death signal.Mol Endocrinol. 2005 Mar;19(3):771-80. doi: 10.1210/me.2004-0161. Epub 2004 Dec 9. Mol Endocrinol. 2005. PMID: 15591537
-
Integrin-mediated signaling in the regulation of osteoclast adhesion and activation.Front Biosci. 1998 Aug 1;3:d757-68. doi: 10.2741/A319. Front Biosci. 1998. PMID: 9682033 Review.
-
The matricellular protein CYR61 inhibits osteoclastogenesis by a mechanism independent of alphavbeta3 and alphavbeta5.Endocrinology. 2007 Dec;148(12):5761-8. doi: 10.1210/en.2007-0473. Epub 2007 Sep 6. Endocrinology. 2007. PMID: 17823253
-
Involvement of alpha(v)beta3 integrins in osteoclast function.J Bone Miner Metab. 2007;25(6):337-44. doi: 10.1007/s00774-007-0773-9. Epub 2007 Oct 25. J Bone Miner Metab. 2007. PMID: 17968485 Review.
Cited by
-
The Role of IGF/IGF-IR-Signaling and Extracellular Matrix Effectors in Bone Sarcoma Pathogenesis.Cancers (Basel). 2021 May 19;13(10):2478. doi: 10.3390/cancers13102478. Cancers (Basel). 2021. PMID: 34069554 Free PMC article. Review.
-
The essential anti-angiogenic strategies in cartilage engineering and osteoarthritic cartilage repair.Cell Mol Life Sci. 2022 Jan 14;79(1):71. doi: 10.1007/s00018-021-04105-0. Cell Mol Life Sci. 2022. PMID: 35029764 Free PMC article. Review.
-
Giantin is required for intracellular N-terminal processing of type I procollagen.J Cell Biol. 2021 Jun 7;220(6):e202005166. doi: 10.1083/jcb.202005166. Epub 2021 May 4. J Cell Biol. 2021. PMID: 33944912 Free PMC article.
-
Positive and negative regulators of osteoclast apoptosis.Bone Rep. 2019 Oct 21;11:100225. doi: 10.1016/j.bonr.2019.100225. eCollection 2019 Dec. Bone Rep. 2019. PMID: 31720316 Free PMC article. Review.
-
Serum levels of PIICP, PIIANP, and PIIBNP are decreased in patients with an endemic osteochondropathy, Kashin-Beck disease.J Orthop Surg Res. 2018 May 29;13(1):128. doi: 10.1186/s13018-018-0840-z. J Orthop Surg Res. 2018. PMID: 29843748 Free PMC article.
References
-
- Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;5:407–13. - PubMed
-
- May H, Murphy S, Khaw KT. Aged--associated Bone Loss in Men and Women and its Relationship to weight. Age Ageing. 1994;23:235–240. - PubMed
-
- Teitelbaum SL. Bone Resorption by Osteoclasts. Science. 2000;289:1504–1508. - PubMed
-
- Manolagas SC. Birth and Death of Bone Cells: Basic Regulatory Mechanisms and Implications for the Pathogenesis and Treatment of Osteoporosis. Endocr Rev. 2000;21:115–137. - PubMed
-
- Kaplan C, Finnegan A. Osteoclasts, pro-inflammatory cytokines, RANK-L and bone remodeling in rheumatoid arthritis. Front Biosci. 2003;8:d1018–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

