Proteomic biomarkers apolipoprotein A1, truncated transthyretin and connective tissue activating protein III enhance the sensitivity of CA125 for detecting early stage epithelial ovarian cancer
- PMID: 21708402
- PMCID: PMC3152646
- DOI: 10.1016/j.ygyno.2011.06.002
Proteomic biomarkers apolipoprotein A1, truncated transthyretin and connective tissue activating protein III enhance the sensitivity of CA125 for detecting early stage epithelial ovarian cancer
Abstract
Objective: The low prevalence of ovarian cancer demands both high sensitivity (>75%) and specificity (99.6%) to achieve a positive predictive value of 10% for successful early detection. Utilizing a two stage strategy where serum marker(s) prompt the performance of transvaginal sonography (TVS) in a limited number (2%) of women could reduce the requisite specificity for serum markers to 98%. We have attempted to improve sensitivity by combining CA125 with proteomic markers.
Methods: Sera from 41 patients with early stage (I/II) and 51 with late stage (III/IV) epithelial ovarian cancer, 40 with benign disease and 99 healthy individuals, were analyzed to measure 7 proteins [Apolipoprotein A1 (Apo-A1), truncated transthyretin (TT), transferrin, hepcidin, ß-2-microglobulin (ß2M), Connective Tissue Activating Protein III (CTAPIII), and Inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4)]. Statistical models were fit by logistic regression, followed by optimization of factors retained in the models determined by optimizing the Akaike Information Criterion. A validation set included 136 stage I ovarian cancers, 140 benign pelvic masses and 174 healthy controls.
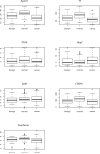
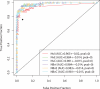
Results: In a training set analysis, the 3 most effective biomarkers (Apo-A1, TT and CTAPIII) exhibited 54% sensitivity at 98% specificity, CA125 alone produced 68% sensitivity and the combination increased sensitivity to 88%. In a validation set, the marker panel plus CA125 produced a sensitivity of 84% at 98% specificity (P=0.015, McNemar's test).
Conclusion: Combining a panel of proteomic markers with CA125 could provide a first step in a sequential two-stage strategy with TVS for early detection of ovarian cancer.
Copyright © 2011. Published by Elsevier Inc.
Figures
Similar articles
-
Proteomic biomarkers in combination with CA 125 for detection of epithelial ovarian cancer using prediagnostic serum samples from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial.Cancer. 2012 Jan 1;118(1):91-100. doi: 10.1002/cncr.26241. Epub 2011 Jun 29. Cancer. 2012. PMID: 21717433 Free PMC article.
-
Highly accurate detection of ovarian cancer using CA125 but limited improvement with serum matrix-assisted laser desorption/ionization time-of-flight mass spectrometry profiling.Int J Gynecol Cancer. 2010 Dec;20(9):1518-24. doi: 10.1111/IGC.0b013e3181fc1990. Int J Gynecol Cancer. 2010. PMID: 21370595
-
Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer.Cancer Res. 2004 Aug 15;64(16):5882-90. doi: 10.1158/0008-5472.CAN-04-0746. Cancer Res. 2004. PMID: 15313933
-
New tumor markers: CA125 and beyond.Int J Gynecol Cancer. 2005 Nov-Dec;15 Suppl 3:274-81. doi: 10.1111/j.1525-1438.2005.00441.x. Int J Gynecol Cancer. 2005. PMID: 16343244 Review.
-
HE4 in the differential diagnosis of ovarian masses.Clin Chim Acta. 2015 Jun 15;446:147-55. doi: 10.1016/j.cca.2015.03.047. Epub 2015 Apr 16. Clin Chim Acta. 2015. PMID: 25892674 Review.
Cited by
-
Serum amyloid P component and pro-platelet basic protein in extracellular vesicles or serum are novel markers of liver fibrosis in chronic hepatitis C patients.PLoS One. 2022 Jul 7;17(7):e0271020. doi: 10.1371/journal.pone.0271020. eCollection 2022. PLoS One. 2022. PMID: 35797333 Free PMC article.
-
CTAPIII/CXCL7: a novel biomarker for early diagnosis of lung cancer.Cancer Med. 2018 Feb;7(2):325-335. doi: 10.1002/cam4.1292. Epub 2018 Jan 22. Cancer Med. 2018. PMID: 29356357 Free PMC article.
-
APOA1 Is a Novel Marker for Preeclampsia.Int J Mol Sci. 2023 Nov 15;24(22):16363. doi: 10.3390/ijms242216363. Int J Mol Sci. 2023. PMID: 38003549 Free PMC article.
-
Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer.Cancers (Basel). 2019 Aug 1;11(8):1097. doi: 10.3390/cancers11081097. Cancers (Basel). 2019. PMID: 31374929 Free PMC article. Review.
-
Glycoproteomic Analysis of Malignant Ovarian Cancer Ascites Fluid Identifies Unusual Glycopeptides.J Proteome Res. 2016 Sep 2;15(9):3358-76. doi: 10.1021/acs.jproteome.6b00548. Epub 2016 Aug 23. J Proteome Res. 2016. PMID: 27500424 Free PMC article.
References
-
- Berek JS, Friedlander ML, Bast RC., Jr . Ovarian Cancer. In: Hong WK, Bast RC Jr, Hait W, Kufe D, Pollock RE, Weichselbaum RR, Holland JF, Frei E III, editors. Holland-Frei Cancer Medicine. 8th Edition PMPH-USA; Shelton, CT: 2010. pp. 1344–1375.
-
- Bast RC, Jr, Klug TL, St. John E, Jenison E, Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker L, Zurawski VR, Jr, Knapp RC. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. New Engl J Med. 1983;309:883–887. - PubMed
-
- Rosen D, Wang L, Atkinson JN, Yu Y, Lu K, Diamandis EP, Hellstrom I, Mok SC, Liu J, Bast RC., Jr Potential markers that complement expression of CA 125 in epithelial ovarian cancer. Gynecol Oncol. 2005;99:267–77. - PubMed
-
- Skates SJ, Xu FJ, Yu YH, Sjovall K, Einhorn N, Chang Y, et al. Toward an optimal algorithm for ovarian cancer screening with longitudinal tumor markers. Cancer. 1995;76(10 Suppl):2004–2010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

