Mitochondria: isolation, structure and function
- PMID: 21708903
- PMCID: PMC3208215
- DOI: 10.1113/jphysiol.2011.212712
Mitochondria: isolation, structure and function
Abstract
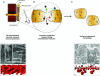
Mitochondria are complex organelles constantly undergoing processes of fusion and fission, processes that not only modulate their morphology, but also their function. Yet the assessment of mitochondrial function in skeletal muscle often involves mechanical isolation of the mitochondria, a process which disrupts their normally heterogeneous branching structure and yields relatively homogeneous spherical organelles. Alternatively, methods have been used where the sarcolemma is permeabilized and mitochondrial morphology is preserved, but both methods face the downside that they remove potential influences of the intracellular milieu on mitochondrial function. Importantly, recent evidence shows that the fragmented mitochondrial morphology resulting from routine mitochondrial isolation procedures used with skeletal muscle alters key indices of function in a manner qualitatively similar to mitochondria undergoing fission in vivo. Although these results warrant caution when interpreting data obtained with mitochondria isolated from skeletal muscle, they also suggest that isolated mitochondrial preparations might present a useful way of interrogating the stress resistance of mitochondria. More importantly, these new findings underscore the empirical value of studying mitochondrial function in minimally disruptive experimental preparations. In this review, we briefly discuss several considerations and hypotheses emerging from this work.
Figures

References
-
- Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol. 2005;289:C994–C1001. - PubMed
-
- Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol. 2006;290:C844–851. - PubMed
-
- Bakeeva LE, Chentsov Yu S, Skulachev VP. Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta. 1978;501:349–369. - PubMed
-
- Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. - PubMed
-
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. VI. The effects of adenosine diphosphate on azide-treated mitochondria. J Biol Chem. 1956;221:477–489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

