B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells
- PMID: 21708925
- PMCID: PMC3135363
- DOI: 10.1084/jem.20102065
B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells
Abstract
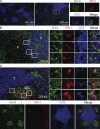
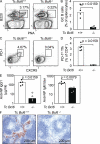
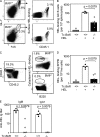
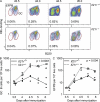
T follicular helper cells (Tfh cells) localize to follicles where they provide growth and selection signals to mutated germinal center (GC) B cells, thus promoting their differentiation into high affinity long-lived plasma cells and memory B cells. T-dependent B cell differentiation also occurs extrafollicularly, giving rise to unmutated plasma cells that are important for early protection against microbial infections. Bcl-6 expression in T cells has been shown to be essential for the formation of Tfh cells and GC B cells, but little is known about its requirement in physiological extrafollicular antibody responses. We use several mouse models in which extrafollicular plasma cells can be unequivocally distinguished from those of GC origin, combined with antigen-specific T and B cells, to show that the absence of T cell-expressed Bcl-6 significantly reduces T-dependent extrafollicular antibody responses. Bcl-6(+) T cells appear at the T-B border soon after T cell priming and before GC formation, and these cells express low amounts of PD-1. Their appearance precedes that of Bcl-6(+) PD-1(hi) T cells, which are found within the GC. IL-21 acts early to promote both follicular and extrafollicular antibody responses. In conclusion, Bcl-6(+) T cells are necessary at B cell priming to form extrafollicular antibody responses, and these pre-GC Tfh cells can be distinguished phenotypically from GC Tfh cells.
Figures


References
-
- Cannons J.L., Qi H., Lu K.T., Dutta M., Gomez-Rodriguez J., Cheng J., Wakeland E.K., Germain R.N., Schwartzberg P.L. 2010. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 32:253–265 10.1016/j.immuni.2010.01.010 - DOI - PMC - PubMed
-
- Chan T.D., Gatto D., Wood K., Camidge T., Basten A., Brink R. 2009. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J. Immunol. 183:3139–3149 10.4049/jimmunol.0901690 - DOI - PubMed
-
- Cunningham A.F., Gaspal F., Serre K., Mohr E., Henderson I.R., Scott-Tucker A., Kenny S.M., Khan M., Toellner K.M., Lane P.J., MacLennan I.C. 2007. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol. 178:6200–6207 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

