E4F1 deficiency results in oxidative stress-mediated cell death of leukemic cells
- PMID: 21708927
- PMCID: PMC3135361
- DOI: 10.1084/jem.20101995
E4F1 deficiency results in oxidative stress-mediated cell death of leukemic cells
Abstract
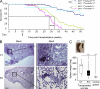
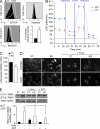
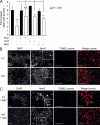
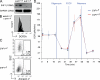
The multifunctional E4F1 protein was originally discovered as a target of the E1A viral oncoprotein. Growing evidence indicates that E4F1 is involved in key signaling pathways commonly deregulated during cell transformation. In this study, we investigate the influence of E4F1 on tumorigenesis. Wild-type mice injected with fetal liver cells from mice lacking CDKN2A, the gene encoding Ink4a/Arf, developed histiocytic sarcomas (HSs), a tumor originating from the monocytic/macrophagic lineage. Cre-mediated deletion of E4F1 resulted in the death of HS cells and tumor regression in vivo and extended the lifespan of recipient animals. In murine and human HS cell lines, E4F1 inactivation resulted in mitochondrial defects and increased production of reactive oxygen species (ROS) that triggered massive cell death. Notably, these defects of E4F1 depletion were observed in HS cells but not healthy primary macrophages. Short hairpin RNA-mediated depletion of E4F1 induced mitochondrial defects and ROS-mediated death in several human myeloid leukemia cell lines. E4F1 protein is overexpressed in a large subset of human acute myeloid leukemia samples. Together, these data reveal a role for E4F1 in the survival of myeloid leukemic cells and support the notion that targeting E4F1 activities might have therapeutic interest.
Figures




References
-
- Carrasco D.R., Fenton T., Sukhdeo K., Protopopova M., Enos M., You M.J., Di Vizio D., Nogueira C., Stommel J., Pinkus G.S., et al. 2006. The PTEN and INK4A/ARF tumor suppressors maintain myelolymphoid homeostasis and cooperate to constrain histiocytic sarcoma development in humans. Cancer Cell. 9:379–390 10.1016/j.ccr.2006.03.028 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

