Vesicle-associated membrane protein-2 (VAMP2) mediates cAMP-stimulated renin release in mouse juxtaglomerular cells
- PMID: 21708949
- PMCID: PMC3151102
- DOI: 10.1074/jbc.M111.225839
Vesicle-associated membrane protein-2 (VAMP2) mediates cAMP-stimulated renin release in mouse juxtaglomerular cells
Abstract
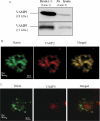
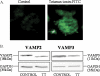
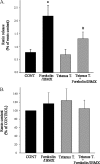
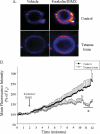
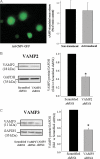
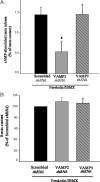
Renin is essential for blood pressure control. Renin is stored in granules in juxtaglomerular (JG) cells, located in the pole of the renal afferent arterioles. The second messenger cAMP stimulates renin release. However, it is unclear whether fusion and exocytosis of renin-containing granules is involved. In addition, the role of the fusion proteins, SNAREs (soluble N-ethylmaleimide-sensitive factor attachment proteins), in renin release from JG cells has not been studied. The vesicle SNARE proteins VAMP2 (vesicle associated membrane protein 2) and VAMP3 mediate cAMP-stimulated exocytosis in other endocrine cells. Thus, we hypothesized that VAMP2 and/or -3 mediate cAMP-stimulated renin release from JG cells. By fluorescence-activated cell sorting, we isolated JG cells expressing green fluorescent protein and compared the relative abundance of VAMP2/3 in JG cells versus total mouse kidney mRNA by quantitative PCR. We found that VAMP2 and VAMP3 mRNA are expressed and enriched in JG cells. Confocal imaging of primary cultures of JG cells showed that VAMP2 (but not VAMP3) co-localized with renin-containing granules. Cleavage of VAMP2 and VAMP3 with tetanus toxin blocked cAMP-stimulated renin release from JG cells by ~50% and impaired cAMP-stimulated exocytosis by ~50%, as monitored with FM1-43. Then we specifically knocked down VAMP2 or VAMP3 by adenoviral-mediated delivery of short hairpin silencing RNA. We found that silencing VAMP2 blocked cAMP-induced renin release by ~50%. In contrast, silencing VAMP3 had no effect on basal or cAMP-stimulated renin release. We conclude that VAMP2 and VAMP3 are expressed in JG cells, but only VAMP2 is targeted to renin-containing granules and mediates the stimulatory effect of cAMP on renin exocytosis.
Figures

Similar articles
-
Regulation of renin secretion by renal juxtaglomerular cells.Pflugers Arch. 2013 Jan;465(1):25-37. doi: 10.1007/s00424-012-1126-7. Epub 2012 Jun 26. Pflugers Arch. 2013. PMID: 22733355 Review.
-
Role of the SNARE protein SNAP23 on cAMP-stimulated renin release in mouse juxtaglomerular cells.Am J Physiol Renal Physiol. 2013 Mar 1;304(5):F498-504. doi: 10.1152/ajprenal.00556.2012. Epub 2012 Dec 26. Am J Physiol Renal Physiol. 2013. PMID: 23269646 Free PMC article.
-
Vesicle-associated membrane protein 2 (VAMP2) but Not VAMP3 mediates cAMP-stimulated trafficking of the renal Na+-K+-2Cl- co-transporter NKCC2 in thick ascending limbs.J Biol Chem. 2014 Aug 22;289(34):23951-62. doi: 10.1074/jbc.M114.589333. Epub 2014 Jul 9. J Biol Chem. 2014. PMID: 25008321 Free PMC article.
-
Parathyroid hormone stimulates juxtaglomerular cell cAMP accumulation without stimulating renin release.Am J Physiol Renal Physiol. 2012 Oct 15;303(8):F1157-65. doi: 10.1152/ajprenal.00269.2012. Epub 2012 Aug 15. Am J Physiol Renal Physiol. 2012. PMID: 22896038 Free PMC article.
-
Renin release: role of SNAREs.Am J Physiol Regul Integr Comp Physiol. 2014 Sep 1;307(5):R484-6. doi: 10.1152/ajpregu.00175.2014. Epub 2014 Jun 18. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24944251 Review.
Cited by
-
Endothelin inhibits renin release from juxtaglomerular cells via endothelin receptors A and B via a transient receptor potential canonical-mediated pathway.Physiol Rep. 2014 Dec 18;2(12):e12240. doi: 10.14814/phy2.12240. Print 2014 Dec 1. Physiol Rep. 2014. PMID: 25524278 Free PMC article.
-
Regulation of renin secretion by renal juxtaglomerular cells.Pflugers Arch. 2013 Jan;465(1):25-37. doi: 10.1007/s00424-012-1126-7. Epub 2012 Jun 26. Pflugers Arch. 2013. PMID: 22733355 Review.
-
Vesicle-associated Membrane Protein 3 (VAMP3) Mediates Constitutive Trafficking of the Renal Co-transporter NKCC2 in Thick Ascending Limbs: ROLE IN RENAL FUNCTION AND BLOOD PRESSURE.J Biol Chem. 2016 Oct 14;291(42):22063-22073. doi: 10.1074/jbc.M116.735167. Epub 2016 Aug 22. J Biol Chem. 2016. PMID: 27551042 Free PMC article.
-
Adenosine inhibits renin release from juxtaglomerular cells via an A1 receptor-TRPC-mediated pathway.Am J Physiol Renal Physiol. 2013 Oct 15;305(8):F1209-19. doi: 10.1152/ajprenal.00710.2012. Epub 2013 Jul 24. Am J Physiol Renal Physiol. 2013. PMID: 23884142 Free PMC article.
-
Macrophages excite muscle spindles with glutamate to bolster locomotion.Nature. 2025 Jan;637(8046):698-707. doi: 10.1038/s41586-024-08272-5. Epub 2024 Dec 4. Nature. 2025. PMID: 39633045 Free PMC article.
References
-
- Gomez R. A., Norwood V. F., Tufro-McReddie A. (1997) Microsc. Res. Tech. 39, 254–260 - PubMed
-
- Sequeira López M. L., Pentz E. S., Nomasa T., Smithies O., Gomez R. A. (2004) Dev. Cell 6, 719–728 - PubMed
-
- Lacombe M. J., Mercure C., Dikeakos J. D., Reudelhuber T. L. (2005) J. Biol. Chem. 280, 4803–4807 - PubMed
-
- Methot D., Reudelhuber T. L. (2001) Curr. Hypertens. Rep. 3, 68–73 - PubMed
-
- Jutras I., Reudelhuber T. L. (1999) FEBS Lett. 443, 48–52 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

