Pak1 regulates focal adhesion strength, myosin IIA distribution, and actin dynamics to optimize cell migration
- PMID: 21708980
- PMCID: PMC3216326
- DOI: 10.1083/jcb.201010059
Pak1 regulates focal adhesion strength, myosin IIA distribution, and actin dynamics to optimize cell migration
Abstract
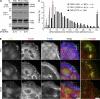
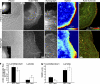
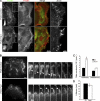
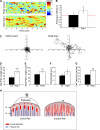
Cell motility requires the spatial and temporal coordination of forces in the actomyosin cytoskeleton with extracellular adhesion. The biochemical mechanism that coordinates filamentous actin (F-actin) assembly, myosin contractility, adhesion dynamics, and motility to maintain the balance between adhesion and contraction remains unknown. In this paper, we show that p21-activated kinases (Paks), downstream effectors of the small guanosine triphosphatases Rac and Cdc42, biochemically couple leading-edge actin dynamics to focal adhesion (FA) dynamics. Quantitative live cell microscopy assays revealed that the inhibition of Paks abolished F-actin flow in the lamella, displaced myosin IIA from the cell edge, and decreased FA turnover. We show that, by controlling the dynamics of these three systems, Paks regulate the protrusive activity and migration of epithelial cells. Furthermore, we found that expressing Pak1 was sufficient to overcome the inhibitory effects of excess adhesion strength on cell motility. These findings establish Paks as critical molecules coordinating cytoskeletal systems for efficient cell migration.
Figures


Similar articles
-
Micro-environmental control of cell migration--myosin IIA is required for efficient migration in fibrillar environments through control of cell adhesion dynamics.J Cell Sci. 2012 May 1;125(Pt 9):2244-56. doi: 10.1242/jcs.098806. Epub 2012 Feb 10. J Cell Sci. 2012. PMID: 22328520 Free PMC article.
-
p21-Activated kinase (Pak) regulates airway smooth muscle contraction by regulating paxillin complexes that mediate actin polymerization.J Physiol. 2016 Sep 1;594(17):4879-900. doi: 10.1113/JP272132. Epub 2016 May 29. J Physiol. 2016. PMID: 27038336 Free PMC article.
-
Non-muscle myosin IIA differentially regulates intestinal epithelial cell restitution and matrix invasion.Am J Pathol. 2009 Feb;174(2):436-48. doi: 10.2353/ajpath.2009.080171. Epub 2009 Jan 15. Am J Pathol. 2009. PMID: 19147824 Free PMC article.
-
Mammalian nonmuscle myosin II comes in three flavors.Biochem Biophys Res Commun. 2018 Nov 25;506(2):394-402. doi: 10.1016/j.bbrc.2018.03.103. Epub 2018 Mar 17. Biochem Biophys Res Commun. 2018. PMID: 29550471 Free PMC article. Review.
-
Mechanical integration of actin and adhesion dynamics in cell migration.Annu Rev Cell Dev Biol. 2010;26:315-33. doi: 10.1146/annurev.cellbio.011209.122036. Annu Rev Cell Dev Biol. 2010. PMID: 19575647 Free PMC article. Review.
Cited by
-
The PAKs come of age: Celebrating 18 years of discovery.Cell Logist. 2012 Apr 1;2(2):54-58. doi: 10.4161/cl.22084. Cell Logist. 2012. PMID: 23125949 Free PMC article.
-
Hyperactivity of Rac1-GTPase pathway impairs neuritogenesis of cortical neurons by altering actin dynamics.Sci Rep. 2018 May 8;8(1):7254. doi: 10.1038/s41598-018-25354-3. Sci Rep. 2018. PMID: 29740022 Free PMC article.
-
Induction of focal adhesions and motility in Drosophila S2 cells.Mol Biol Cell. 2014 Dec 1;25(24):3861-9. doi: 10.1091/mbc.E14-04-0863. Epub 2014 Oct 1. Mol Biol Cell. 2014. PMID: 25273555 Free PMC article.
-
Dysregulation of PAK1 Is Associated with DNA Damage and Is of Prognostic Importance in Primary Esophageal Small Cell Carcinoma.Int J Mol Sci. 2015 May 27;16(6):12035-50. doi: 10.3390/ijms160612035. Int J Mol Sci. 2015. PMID: 26023713 Free PMC article.
-
Integrin-mediated cell surface recruitment of autotaxin promotes persistent directional cell migration.FASEB J. 2014 Feb;28(2):861-70. doi: 10.1096/fj.13-232868. Epub 2013 Nov 25. FASEB J. 2014. PMID: 24277575 Free PMC article.
References
-
- Adams M.C., Matov A., Yarar D., Gupton S.L., Danuser G., Waterman-Storer C.M. 2004. Signal analysis of total internal reflection fluorescent speckle microscopy (TIR-FSM) and wide-field epi-fluorescence FSM of the actin cytoskeleton and focal adhesions in living cells. J. Microsc. 216:138–152 10.1111/j.0022-2720.2004.01408.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

