Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding
- PMID: 21708990
- PMCID: PMC3165461
- DOI: 10.1128/IAI.05182-11
Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding
Abstract
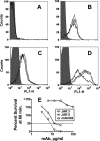
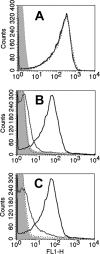
Binding of the complement-downregulating protein factor H (fH) to the surface of the meningococcus is important for survival of the organism in human serum. The meningococcal vaccine candidate factor H binding protein (fHbp) is an important ligand for human fH. While some fHbp-specific monoclonal antibodies (MAbs) block binding of fH to fHbp, the stoichiometry of blocking in the presence of high serum concentrations of fH and its effect on complement-mediated bactericidal activity are unknown. To investigate this question, we constructed chimeric antibodies in which the human IgG1 constant region was paired with three murine fHbp-specific binding domains designated JAR 3, JAR 5, and MAb502. By surface plasmon resonance, the association rates for binding of all three MAbs to immobilized fHbp were >50-fold higher than that for binding of fH to fHbp, and the MAb dissociation rates were >500-fold lower than that for fH. While all three MAbs elicited similar C1q-dependent C4b deposition on live bacteria (classical complement pathway), only those antibodies that inhibited binding of fH to fHbp (JAR 3 and JAR 5) had bactericidal activity with human complement. MAb502, which did not inhibit fH binding, had complement-mediated bactericidal activity only when tested with fH-depleted human complement. When an IgG1 anti-fHbp MAb binds to sparsely exposed fHbp on the bacterial surface, there appears to be insufficient complement activation for bacteriolysis unless fH binding also is inhibited. The ability of fHbp vaccines to elicit protective antibodies, therefore, is likely to be enhanced if the antibody repertoire is of high avidity and includes fH-blocking activity.
Figures

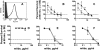
References
-
- Bambini S., et al. 2009. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine 27:2794–2803 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

