Context-dependent activation kinetics elicited by soluble versus outer membrane vesicle-associated heat-labile enterotoxin
- PMID: 21708992
- PMCID: PMC3165487
- DOI: 10.1128/IAI.05336-11
Context-dependent activation kinetics elicited by soluble versus outer membrane vesicle-associated heat-labile enterotoxin
Abstract
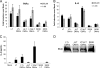
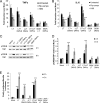
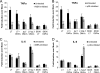
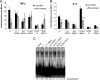
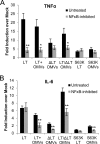
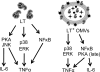
Enterotoxigenic Escherichia coli (ETEC) is the leading cause of traveler's diarrhea and children's diarrhea worldwide. Among its virulence factors, ETEC produces heat-labile enterotoxin (LT). Most secreted LT is associated with outer membrane vesicles that are rich in lipopolysaccharide. The majority of prior studies have focused on soluble LT purified from ETEC periplasm. We investigated the hypothesis that the extracellular vesicle context of toxin presentation might be important in eliciting immune responses. We compared the polarized epithelial cell responses to apically applied soluble LT and LT-containing vesicles (LT(+) vesicles) as well as controls using a catalytically inactive mutant of LT and vesicles lacking LT. Although vesicle treatments with no or catalytically inactive LT induced a modest amount of interleukin-6 (IL-6), samples containing catalytically active LT elicited higher levels. A combination of soluble LT and LT-deficient vesicles induced significantly higher IL-6 levels than either LT or LT(+) vesicles alone. The responses to LT(+) vesicles were found to be independent of the canonical LT pathway, because the inhibition of cyclic AMP response element (CRE)-binding protein (CREB) phosphorylation did not lead to a decrease in cytokine gene expression levels. Furthermore, soluble LT caused earlier phosphorylation of CREB and activation of CRE compared with LT(+) vesicles. Soluble LT also led to the activation of activator protein 1, whereas LT(+) vesicle IL-6 responses appeared to be mediated by NF-κB. In summary, the results demonstrate that soluble LT and vesicle-bound LT elicit ultimately similar cytokine responses through distinct different activation pathways.
Figures
References
-
- Artis D. 2008. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 8:411–420 - PubMed
-
- Ashwell J. D. 2006. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 6:532–540 - PubMed
-
- Black R. 1990. Epidemiology of travelers' diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12:S73–S79 - PubMed
-
- Cheng E., Cardenas L., Clements J. D. 1999. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine 18:38–49 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

