Caspase-1 activation of interleukin-1β (IL-1β) and IL-18 is dispensable for induction of experimental cerebral malaria
- PMID: 21708993
- PMCID: PMC3165484
- DOI: 10.1128/IAI.05459-11
Caspase-1 activation of interleukin-1β (IL-1β) and IL-18 is dispensable for induction of experimental cerebral malaria
Abstract
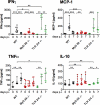
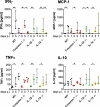
Malaria infection is initiated by sporozoite invasion of hepatocytes and asexual reproduction of liver stages, processes that are regarded to be "clinically and diagnostically silent." Merozoites, which egress from hepatocytes, infect erythrocytes in periodic cycles and induce disease. How the host innate immune system contributes to disease outcomes and to the induction of effector cells during malaria remains unclear. Likewise, how the initial liver stages may shape responses to blood-stage parasites is unknown. Here, using both sporozoite- and blood-stage-induced infections with the rodent malaria parasite Plasmodium berghei ANKA, we show that the MyD88 and Toll-like receptor 2/4 (TLR2/4) pathways play critical roles in the development of experimental cerebral malaria (ECM). Strikingly, an absolute dependence on MyD88 and TLR2/4 was observed when infections were initiated with sporozoites. In addition, we show that caspase-1 activation of interleukin-1β (IL-1β) and IL-18, which is associated with the inflammasome pathway, does not contribute to P. berghei ANKA-induced immunopathology. Consistent with these data, prophylactic cover with the IL-1β antagonist anakinra did not reduce the incidence of ECM. Therefore, we propose that protection against ECM due to loss of TLR signaling functions is caused by effector mechanisms other than IL-1β activation.
Figures




References
-
- Adachi O., et al. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150 - PubMed
-
- Adams S., Brown H., Turner G. 2002. Breaking down the blood-brain barrier: signaling a path to cerebral malaria? Trends Parasitol. 18:360–366 - PubMed
-
- Amani V., et al. 2000. Involvement of IFN-gamma receptor-medicated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur. J. Immunol. 30:1646–1655 - PubMed
-
- Bazan J. F., Timans J. C., Kastelein R. A. 1996. A newly defined interleukin-1? Nature 379:591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

