Allelic variation of the Lyme disease spirochete adhesin DbpA influences spirochetal binding to decorin, dermatan sulfate, and mammalian cells
- PMID: 21708995
- PMCID: PMC3165495
- DOI: 10.1128/IAI.00163-11
Allelic variation of the Lyme disease spirochete adhesin DbpA influences spirochetal binding to decorin, dermatan sulfate, and mammalian cells
Abstract
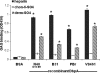
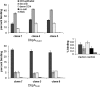
After transmission by an infected tick, the Lyme disease spirochete, Borrelia burgdorferi sensu lato, colonizes the mammalian skin and may disseminate systemically. The three major species of Lyme disease spirochete--B. burgdorferi sensu stricto, B. garinii, and B. afzelii--are associated with different chronic disease manifestations. Colonization is likely promoted by the ability to bind to target tissues, and Lyme disease spirochetes utilize multiple adhesive molecules to interact with diverse mammalian components. The allelic variable surface lipoprotein decorin binding protein A (DbpA) promotes bacterial binding to the proteoglycan decorin and to the glycosaminoglycan (GAG) dermatan sulfate. To assess allelic variation of DbpA in GAG-, decorin-, and cell-binding activities, we expressed dbpA alleles derived from diverse Lyme disease spirochetes in B. burgdorferi strain B314, a noninfectious and nonadherent strain that lacks dbpA. Each DbpA allele conferred upon B. burgdorferi strain B314 the ability to bind to cultured kidney epithelial (but not glial or endothelial) cells, as well as to purified decorin and dermatan sulfate. Nevertheless, allelic variation of DbpA was associated with dramatic differences in substrate binding activity. In most cases, decorin and dermatan sulfate binding correlated well, but DbpA of B. afzelii strain VS461 promoted differential binding to decorin and dermatan sulfate, indicating that the two activities are separable. DbpA from a clone of B. burgdorferi strain N40 that can cause disseminated infection in mice displayed relatively low adhesive activity, indicating that robust DbpA-mediated adhesive activity is not required for spread in the mammalian host.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

