Melatonin attenuates colistin-induced nephrotoxicity in rats
- PMID: 21709095
- PMCID: PMC3165279
- DOI: 10.1128/AAC.00328-11
Melatonin attenuates colistin-induced nephrotoxicity in rats
Abstract
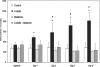
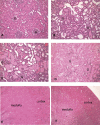
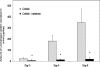
Colistin-induced nephrotoxicity is a dose-limiting adverse effect when colistin is used against Gram-negative pathogens. This study examined the nephroprotective effect of melatonin against colistin in rats. Rats (n = 7 per group) were treated intravenously twice daily with saline, colistin (at increasing doses from 0.5 to 4.0 mg/kg), melatonin (5 mg/kg), or both melatonin and colistin for 7 days. The severity of renal alteration was examined both biochemically and histologically. The effect of coadministration of melatonin on colistin pharmacokinetics was investigated. Significantly lower urinary N-acetyl-β-d-glucosaminidase excretion was observed from day 1 in the colistin-melatonin group compared to the colistin group (P < 0.0001). Plasma creatinine increased significantly (P = 0.023) only in the colistin group on day 6. Significant histological abnormalities (P < 0.0001) were detected only in the kidneys of the colistin group. Melatonin altered colistin pharmacokinetics; the total body clearance in the colistin-melatonin group (1.82 ± 0.26 ml/min/kg) was lower than in the colistin group (4.28 ± 0.93 ml/min/kg). This is the first study demonstrating the protective effect of melatonin against colistin-induced nephrotoxicity, which indicates that colistin-induced nephrotoxicity is mediated through oxidative stress. It also highlights the potential of coadministering an antioxidant to widen the therapeutic window of this very important last-line antibiotic.
Figures

References
-
- Antoniadou A., et al. 2007. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J. Antimicrob. Chemother. 59:786–790 - PubMed
-
- Beno P., Krcmery V., Demitrovicova A. 2006. Bacteremia in cancer patients caused by colistin-resistant Gram-negative bacilli after previous exposure to ciprofloxacin and/or colistin. Clin. Microbiol. Infect. 12:497–498 - PubMed
-
- Celik I., Cihangiroglu M., Ilhan N., Akpolat N., Akbulut H. H. 2005. Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic Clin. Pharmacol. Toxicol. 97:325–332 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

