Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner
- PMID: 21709104
- PMCID: PMC3165298
- DOI: 10.1128/AAC.00583-11
Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner
Abstract
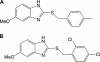
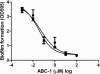
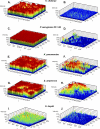
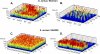
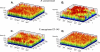
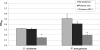
Bacterial biofilm formation causes significant industrial economic loss and high morbidity and mortality in medical settings. Biofilms are defined as multicellular communities of bacteria encased in a matrix of protective extracellular polymers. Because biofilms have a high tolerance for treatment with antimicrobials, protect bacteria from immune defense, and resist clearance with standard sanitation protocols, it is critical to develop new approaches to prevent biofilm formation. Here, a novel benzimidazole molecule, named antibiofilm compound 1 (ABC-1), identified in a small-molecule screen, was found to prevent bacterial biofilm formation in multiple Gram-negative and Gram-positive bacterial pathogens, including Pseudomonas aeruginosa and Staphylococcus aureus, on a variety of different surface types. Importantly, ABC-1 itself does not inhibit the growth of bacteria, and it is effective at nanomolar concentrations. Also, coating a polystyrene surface with ABC-1 reduces biofilm formation. These data suggest ABC-1 is a new chemical scaffold for the development of antibiofilm compounds.
Figures

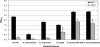

Similar articles
-
Inhibitory effects of antibiofilm compound 1 against Staphylococcus aureus biofilms.Microbiol Immunol. 2016 Mar;60(3):148-59. doi: 10.1111/1348-0421.12359. Microbiol Immunol. 2016. PMID: 26786482
-
Antibiofilm activity of Fmoc-phenylalanine against Gram-positive and Gram-negative bacterial biofilms.J Antibiot (Tokyo). 2021 Jun;74(6):407-416. doi: 10.1038/s41429-021-00409-2. Epub 2021 Feb 26. J Antibiot (Tokyo). 2021. PMID: 33637856
-
Induction of amylase and protease as antibiofilm agents by starch, casein, and yeast extract in Arthrobacter sp. CW01.BMC Microbiol. 2021 Aug 23;21(1):232. doi: 10.1186/s12866-021-02294-z. BMC Microbiol. 2021. PMID: 34425755 Free PMC article.
-
[Advances in the study of synergistic effect of anti-biofilm agents].Yao Xue Xue Bao. 2012 Mar;47(3):339-45. Yao Xue Xue Bao. 2012. PMID: 22645757 Review. Chinese.
-
Antibiofilm Peptides: Potential as Broad-Spectrum Agents.J Bacteriol. 2016 Sep 9;198(19):2572-8. doi: 10.1128/JB.00017-16. Print 2016 Oct 1. J Bacteriol. 2016. PMID: 27068589 Free PMC article. Review.
Cited by
-
Water-Assisted Self-Aggregation of Benzimidazole and Triazole Adducts.ACS Omega. 2019 Jan 7;4(1):437-443. doi: 10.1021/acsomega.8b02688. eCollection 2019 Jan 31. ACS Omega. 2019. PMID: 31459341 Free PMC article.
-
Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal.Folia Microbiol (Praha). 2018 Jul;63(4):413-432. doi: 10.1007/s12223-018-0585-4. Epub 2018 Jan 19. Folia Microbiol (Praha). 2018. PMID: 29352409 Review.
-
Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment.Microorganisms. 2023 Jun 19;11(6):1614. doi: 10.3390/microorganisms11061614. Microorganisms. 2023. PMID: 37375116 Free PMC article. Review.
-
Tryptophan inhibits biofilm formation by Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2013 Apr;57(4):1921-5. doi: 10.1128/AAC.00007-13. Epub 2013 Jan 14. Antimicrob Agents Chemother. 2013. PMID: 23318791 Free PMC article.
-
Efficient Double Suzuki Cross-Coupling Reactions of 2,5-Dibromo-3-hexylthiophene: Anti-Tumor, Haemolytic, Anti-Thrombolytic and Biofilm Inhibition Studies.Molecules. 2016 Jul 27;21(8):977. doi: 10.3390/molecules21080977. Molecules. 2016. PMID: 27472312 Free PMC article.
References
-
- Achar K. C., Hosamani K. M., Seetharamareddy H. R. 2010. In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur. J. Med. Chem. 45:2048–2054 - PubMed
-
- Agle M. E., Martin S. E., Blaschek H. P. 2005. Survival of Shigella boydii 18 in bean salad. J. Food Prot. 68:838–840 - PubMed
-
- Chan Y. C., Blaschek H. P. 2005. Comparative analysis of Shigella boydii 18 foodborne outbreak isolate and related enteric bacteria: role of rpoS and adiA in acid stress response. J. Food Prot. 68:521–527 - PubMed
-
- Chung A. J., Rubner M. F. 2002. Methods of loading and releasing low molecular weight cationic molecules in weak polyelectrolyte multilayer films. Langmuir 18:1176–1183
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

