Cytosolic DNA-activated human dendritic cells are potent activators of the adaptive immune response
- PMID: 21709148
- PMCID: PMC3140546
- DOI: 10.4049/jimmunol.1100469
Cytosolic DNA-activated human dendritic cells are potent activators of the adaptive immune response
Abstract
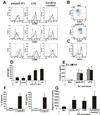
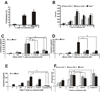
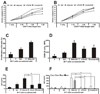
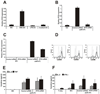
Recent studies in cell lines and genetically engineered mice have demonstrated that cytosolic dsDNA could activate dendritic cells (DCs) to become effector APCs. Recognition of DNA might be a major factor in antimicrobial immune responses against cytosolic pathogens and also in human autoimmune diseases such as systemic lupus erythematosus. However, the role of cytosolic dsDNA in human DC activation and its effects on effector T and B cells are still elusive. In this study, we demonstrate that intracellular dsDNA is a potent activator of human monocyte-derived DCs as well as primary DCs. Activation by dsDNA depends on NF-κB activation, partially on the adaptor molecule IFN-promoter stimulator-1 and the novel cytosolic dsDNA receptor IFI16, but not on the previously recognized dsDNA sentinels absent in melanoma 2, DNA-dependent activator of IFN regulatory factor 3, RNA polymerase III, or high-mobility group boxes. More importantly, we report for the first time, to our knowledge, that human dsDNA-activated DCs, rather than LPS- or inflammatory cytokine mixture-activated DCs, represent the most potent inducers of naive CD4(+) T cells to promote Th1-type cytokine production and generate CD4(+) and CD8(+) cytotoxic T cells. dsDNA-DCs, but not LPS- or mixture-activated DCs, induce B cells to produce complement-fixing IgG1 and IgG3 Abs. We propose that cytosolic dsDNA represents a novel, more effective approach to generate DCs to enhance vaccine effectiveness in reprogramming the adaptive immune system to eradicate infectious agents, autoimmunity, allergy, and cancer.
Figures


References
-
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767. - PubMed
-
- Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291. - PubMed
-
- Kaisho T, Akira S. Critical roles of Toll-like receptors in host defense. Crit Rev Immunol. 2000;20:393. - PubMed
-
- Jefferies CA, Fitzgerald KA. Interferon gene regulation: not all roads lead to Tolls. Trends Mol Med. 2005;11:403. - PubMed
-
- Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

