TNF receptor 1 mediates dendritic cell maturation and CD8 T cell response through two distinct mechanisms
- PMID: 21709152
- PMCID: PMC3380805
- DOI: 10.4049/jimmunol.1002902
TNF receptor 1 mediates dendritic cell maturation and CD8 T cell response through two distinct mechanisms
Abstract
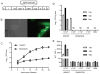
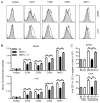
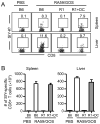
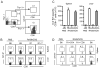
TNF-α and its two receptors (TNFR1 and 2) are known to stimulate dendritic cell (DC) maturation and T cell response. However, the specific receptor and mechanisms involved in vivo are still controversial. In this study, we show that in response to an attenuated mouse hepatitis virus infection, DCs fail to mobilize and up-regulate CD40, CD80, CD86, and MHC class I in TNFR1(-/-) mice as compared with the wild-type and TNFR2(-/-) mice. Correspondingly, virus-specific CD8 T cell response was dramatically diminished in TNFR1(-/-) mice. Adoptive transfer of TNFR1-expressing DCs into TNFR1(-/-) mice rescues CD8 T cell response. Interestingly, adoptive transfer of TNFR1-expressing naive T cells also restores DC mobilization and maturation and endogenous CD8 T cell response. These results show that TNFR1, not TNFR2, mediates TNF-α stimulation of DC maturation and T cell response to mouse hepatitis virus in vivo. They also suggest two mechanisms by which TNFR1 mediates TNF-α-driven DC maturation, as follows: a direct effect through TNFR1 expressed on immature DCs and an indirect effect through TNFR1 expressed on naive T cells.
Figures


References
-
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. - PubMed
-
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. - PubMed
-
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

