Developmental control of oocyte maturation and egg activation in metazoan models
- PMID: 21709181
- PMCID: PMC3179337
- DOI: 10.1101/cshperspect.a005553
Developmental control of oocyte maturation and egg activation in metazoan models
Abstract
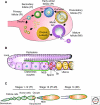
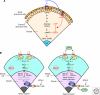
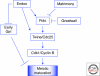
Production of functional eggs requires meiosis to be coordinated with developmental signals. Oocytes arrest in prophase I to permit oocyte differentiation, and in most animals, a second meiotic arrest links completion of meiosis to fertilization. Comparison of oocyte maturation and egg activation between mammals, Caenorhabditis elegans, and Drosophila reveal conserved signaling pathways and regulatory mechanisms as well as unique adaptations for reproductive strategies. Recent studies in mammals and C. elegans show the role of signaling between surrounding somatic cells and the oocyte in maintaining the prophase I arrest and controlling maturation. Proteins that regulate levels of active Cdk1/cyclin B during prophase I arrest have been identified in Drosophila. Protein kinases play crucial roles in the transition from meiosis in the oocyte to mitotic embryonic divisions in C. elegans and Drosophila. Here we will contrast the regulation of key meiotic events in oocytes.
Figures

References
-
- Abdu U, Brodsky M, Schupbach T 2002. Activation of a meiotic checkpoint during Drosophila oogenesis regulates the translation of Gurken through Chk2/Mnk. Curr Biol 12: 1645–1651 - PubMed
-
- Abrieu A, Brassac T, Galas S, Fisher D, Labbe JC, Doree M 1998. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci 111: 1751–1757 - PubMed
-
- Alphey L, Jimenez J, White-Cooper H, Dawson I, Nurse P, Glover DM 1992. twine, a cdc25 homolog that functions in the male and female germline of Drosophila. Cell 69: 977–988 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
