Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women's health initiative randomized controlled trial
- PMID: 21709199
- PMCID: PMC3157967
- DOI: 10.1200/JCO.2011.34.5967
Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women's health initiative randomized controlled trial
Abstract
Purpose: In light of inverse relationships reported in observational studies of vitamin D intake and serum 25-hydroxyvitamin D levels with risk of nonmelanoma skin cancer (NMSC) and melanoma, we evaluated the effects of vitamin D combined with calcium supplementation on skin cancer in a randomized placebo-controlled trial.
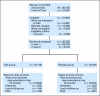
Methods: Postmenopausal women age 50 to 79 years (N = 36,282) enrolled onto the Women's Health Initiative (WHI) calcium/vitamin D clinical trial were randomly assigned to receive 1,000 mg of elemental calcium plus 400 IU of vitamin D3 (CaD) daily or placebo for a mean follow-up period of 7.0 years. NMSC and melanoma skin cancers were ascertained by annual self-report; melanoma skin cancers underwent physician adjudication.
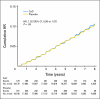
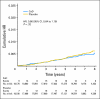
Results: Neither incident NMSC nor melanoma rates differed between treatment (hazard ratio [HR], 1.02; 95% CI, 0.95 to 1.07) and placebo groups (HR, 0.86; 95% CI, 0.64 to 1.16). In subgroup analyses, women with history of NMSC assigned to CaD had a reduced risk of melanoma versus those receiving placebo (HR, 0.43; 95% CI, 0.21 to 0.90; P(interaction) = .038), which was not observed in women without history of NMSC.
Conclusion: Vitamin D supplementation at a relatively low dose plus calcium did not reduce the overall incidence of NMSC or melanoma. However, in women with history of NMSC, CaD supplementation reduced melanoma risk, suggesting a potential role for calcium and vitamin D supplements in this high-risk group. Results from this post hoc subgroup analysis should be interpreted with caution but warrant additional investigation.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: Incidence. J Am Acad Dermatol. 1994;30:774–778. - PubMed
-
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–690. - PubMed
-
- Chen JG, Fleischer AB, Jr, Smith ED, et al. Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg. 2001;27:1035–1038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

