Sugar-regulated cation channel formed by an insect gustatory receptor
- PMID: 21709218
- PMCID: PMC3136286
- DOI: 10.1073/pnas.1019622108
Sugar-regulated cation channel formed by an insect gustatory receptor
Abstract
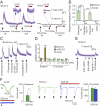
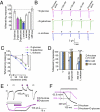
Insects sense the taste of foods and toxic compounds in their environment through the gustatory system. Genetic studies using fruit flies have suggested that putative seven-transmembrane gustatory receptors (Grs) expressed in gustatory sensory neurons are required for responses to specific tastants. We reconstituted sugar responses of Bombyx mori Gr-9 (BmGr-9), a silkworm Gr, in two heterologous expression systems. Xenopus oocytes or HEK293T cells expressing BmGr-9 selectively responded to D-fructose with an influx of extracellular Ca(2+) and a nonselective cation current conductance in a G protein-independent manner. Outside-out patch-clamp recording of BmGr-9-expressing cell membranes provides evidence supporting the hypothesis that BmGr-9 constitutes a ligand-gated ion channel. The fructose-activated current associated with BmGr-9 was suppressed by other hexoses, including glucose and sorbose. The activation and inhibition of insect Gr ion channels may be the molecular basis for the decoding system that discriminates subtle differences in sweet taste. Finally, Drosophila melanogaster Gr43a (DmGr43a), a BmGr-9 ortholog, also responded to D-fructose, suggesting that DmGr43a relatives appear to compose the family of fructose receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. - PubMed
-
- Hill CA, et al. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. - PubMed
-
- Wanner KW, Robertson HM. The gustatory receptor family in the silkworm moth Bombyx mori is characterized by a large expansion of a single lineage of putative bitter receptors. Insect Mol Biol. 2008;17:621–629. - PubMed
-
- Ueno K, et al. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr Biol. 2001;11:1451–1455. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

