Major source of antigenic peptides for the MHC class I pathway is produced during the pioneer round of mRNA translation
- PMID: 21709220
- PMCID: PMC3136330
- DOI: 10.1073/pnas.1104104108
Major source of antigenic peptides for the MHC class I pathway is produced during the pioneer round of mRNA translation
Abstract
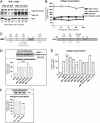
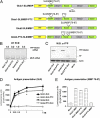
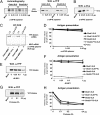
The MHC class I antigen presentation pathway allows the immune system to distinguish between self and nonself. Despite extensive research on the processing of antigenic peptides, little is known about their origin. Here, we show that mRNAs carrying premature stop codons that prevent the production of full-length proteins via the nonsense-mediated decay pathway still produce a majority of peptide substrates for the MHC class I pathway by a noncanonical mRNA translation process. Blocking the interaction of the translation initiation factor eIF4E with the cap structure suppresses the synthesis of full-length proteins but has only a limited effect on the production of antigenic peptides. These results reveal an essential cell biological function for a class of translation products derived during the pioneer round of mRNA translation and will have important implications for understanding how the immune system detects cells harboring pathogens and generates tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rock KL, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. - PubMed
-
- Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat Immunol. 2004;5:670–677. - PubMed
-
- Kloetzel PM. Generation of major histocompatibility complex class I antigens: Functional interplay between proteasomes and TPPII. Nat Immunol. 2004;5:661–669. - PubMed
-
- Serwold T, Gonzalez F, Kim J, Jacob R, Shastri N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419:480–483. - PubMed
-
- Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992;71:1205–1214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

