Myosin-II inhibition and soft 2D matrix maximize multinucleation and cellular projections typical of platelet-producing megakaryocytes
- PMID: 21709232
- PMCID: PMC3136287
- DOI: 10.1073/pnas.1017474108
Myosin-II inhibition and soft 2D matrix maximize multinucleation and cellular projections typical of platelet-producing megakaryocytes
Abstract
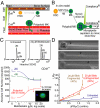
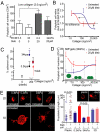
Cell division, membrane rigidity, and strong adhesion to a rigid matrix are all promoted by myosin-II, and so multinucleated cells with distended membranes--typical of megakaryocytes (MKs)--seem predictable for low myosin activity in cells on soft matrices. Paradoxically, myosin mutations lead to defects in MKs and platelets. Here, reversible inhibition of myosin-II is sustained over several cell cycles to produce 3- to 10-fold increases in polyploid MK and a number of other cell types. Even brief inhibition generates highly distensible, proplatelet-like projections that fragment readily under shear, as seen in platelet generation from MKs in vivo. The effects are maximized with collagenous matrices that are soft and 2D, like the perivascular niches in marrow rather than 3D or rigid, like bone. Although multinucleation of other primary hematopoietic lineages helps to generalize a failure-to-fission mechanism, lineage-specific signaling with increased polyploidy proves possible and novel with phospho-regulation of myosin-II heavy chain. Label-free mass spectrometry quantitation of the MK proteome uses a unique proportional peak fingerprint (ProPF) analysis to also show upregulation of the cytoskeletal and adhesion machinery critical to platelet function. Myosin-inhibited MKs generate more platelets in vitro and also in vivo from the marrows of xenografted mice, while agonist stimulation activates platelet spreading and integrin αIIbβ3. Myosin-II thus seems a central, matrix-regulated node for MK-poiesis and platelet generation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Straight AF, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. - PubMed
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
-
- Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279:41263–41266. - PubMed
-
- De Lozanne A, Spudich JA. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236:1086–1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

