Adjacent visual representations of self-motion in different reference frames
- PMID: 21709244
- PMCID: PMC3136271
- DOI: 10.1073/pnas.1102984108
Adjacent visual representations of self-motion in different reference frames
Abstract
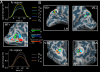
Recent investigations indicate that retinal motion is not directly available for perception when moving around [Souman JL, et al. (2010) J Vis 10:14], possibly pointing to suppression of retinal speed sensitivity in motion areas. Here, we investigated the distribution of retinocentric and head-centric representations of self-rotation in human lower-tier visual motion areas. Functional MRI responses were measured to a set of visual self-motion stimuli with different levels of simulated gaze and simulated head rotation. A parametric generalized linear model analysis of the blood oxygen level-dependent responses revealed subregions of accessory V3 area, V6(+) area, middle temporal area, and medial superior temporal area that were specifically modulated by the speed of the rotational flow relative to the eye and head. Pursuit signals, which link the two reference frames, were also identified in these areas. To our knowledge, these results are the first demonstration of multiple visual representations of self-motion in these areas. The existence of such adjacent representations points to early transformations of the reference frame for visual self-motion signals and a topography by visual reference frame in lower-order motion-sensitive areas. This suggests that visual decisions for action and perception may take into account retinal and head-centric motion signals according to task requirements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Colby CL. Action-oriented spatial reference frames in cortex. Neuron. 1998;20:15–24. - PubMed
-
- Britten KH. Cortical processing of visual motion. In: Albright T, Maslan R, editors. The Senses: A Comprehensive Reference. London: Elsevier; 2008.
-
- Warren WH, Hannon DJ. Direction of self-motion is perceived from optical-flow. Nature. 1988;336:162–163.
-
- van den Berg AV, Beintema JA. The mechanism of interaction between visual flow and eye velocity signals for heading perception. Neuron. 2000;26:747–752. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

