Membrane tension regulates motility by controlling lamellipodium organization
- PMID: 21709265
- PMCID: PMC3136290
- DOI: 10.1073/pnas.1010481108
Membrane tension regulates motility by controlling lamellipodium organization
Abstract
Many cell movements proceed via a crawling mechanism, where polymerization of the cytoskeletal protein actin pushes out the leading edge membrane. In this model, membrane tension has been seen as an impediment to filament growth and cell motility. Here we use a simple model of cell motility, the Caenorhabditis elegans sperm cell, to test how membrane tension affects movement and cytoskeleton dynamics. To enable these analyses, we create transgenic worm strains carrying sperm with a fluorescently labeled cytoskeleton. Via osmotic shock and deoxycholate treatments, we relax or tense the cell membrane and quantify apparent membrane tension changes by the membrane tether technique. Surprisingly, we find that membrane tension reduction is correlated with a decrease in cell displacement speed, whereas an increase in membrane tension enhances motility. We further demonstrate that apparent polymerization rates follow the same trends. We observe that membrane tension reduction leads to an unorganized, rough lamellipodium, composed of short filaments angled away from the direction of movement. On the other hand, an increase in tension reduces lateral membrane protrusions in the lamellipodium, and filaments are longer and more oriented toward the direction of movement. Overall we propose that membrane tension optimizes motility by streamlining polymerization in the direction of movement, thus adding a layer of complexity to our current understanding of how membrane tension enters into the motility equation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



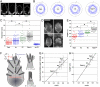
 , red arrow) corresponds to the projection (dotted line) of the polymerization flux (vp, black arrow) along the cell axis and decreases with increasing filament misalignment (angle θ) according to the relation 〈vapp〉 = vp〈 cos θ〉. The energy cost of membrane deformation around finger-like protrusions is reduced when neighboring protrusions align with one another (see text). (G) Plot of average apparent polymerization speeds versus average cos θ under four membrane tension conditions. All values +/− SD. The linear correlation is 0.97, with Hyper++ on the curve upon full extrapolation of the error bars in both the x and y directions. (H) Plot of average filament length versus average cos θ under four membrane tension conditions. All values +/− SD. All length differences between the different conditions are significantly different. The linear correlation is 0.97. In A and D, bar = 5 μm.
, red arrow) corresponds to the projection (dotted line) of the polymerization flux (vp, black arrow) along the cell axis and decreases with increasing filament misalignment (angle θ) according to the relation 〈vapp〉 = vp〈 cos θ〉. The energy cost of membrane deformation around finger-like protrusions is reduced when neighboring protrusions align with one another (see text). (G) Plot of average apparent polymerization speeds versus average cos θ under four membrane tension conditions. All values +/− SD. The linear correlation is 0.97, with Hyper++ on the curve upon full extrapolation of the error bars in both the x and y directions. (H) Plot of average filament length versus average cos θ under four membrane tension conditions. All values +/− SD. All length differences between the different conditions are significantly different. The linear correlation is 0.97. In A and D, bar = 5 μm.References
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

