Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential
- PMID: 21710468
- PMCID: PMC3403816
- DOI: 10.1002/stem.680
Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential
Abstract
The airway epithelium is in direct contact with the environment and therefore constantly at risk for injury. Basal cells (BCs) have been found to repair the surface epithelium (SE), but the contribution of other stem cell populations to airway epithelial repair has not been identified. We demonstrated that airway submucosal gland (SMG) duct cells, in addition to BCs, survived severe hypoxic-ischemic injury. We developed a method to isolate duct cells from the airway. In vitro and in vivo models were used to compare the self-renewal and differentiation potential of duct cells and BCs. We found that only duct cells were capable of regenerating SMG tubules and ducts, as well as the SE overlying the SMGs. SMG duct cells are therefore a multipotent stem cell for airway epithelial repair This is of importance to the field of lung regeneration as determining the repairing cell populations could lead to the identification of novel therapeutic targets and cell-based therapies for patients with airway diseases.
Copyright © 2011 AlphaMed Press.
Conflict of interest statement
There are no conflicts of interest.
Figures

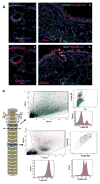


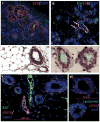
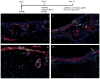
References
-
- Bartlett JA, Fischer AJ, McCray PB., Jr Innate immune functions of the airway epithelium. Contrib Microbiol. 2008;15:147–163. - PubMed
-
- Schoch KG, Lori A, Burns KA, et al. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol. 2004;286:L631–L642. - PubMed
-
- Hong KU, Reynolds SD, Watkins S, et al. In vivo differentiation potential of tracheal basal cells: Evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–L649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

