Carotenoid radical formation: dependence on conjugation length
- PMID: 21711000
- PMCID: PMC3171803
- DOI: 10.1021/jp204787b
Carotenoid radical formation: dependence on conjugation length
Abstract
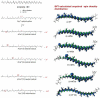
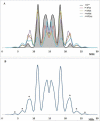
The relative energy of carotenoid neutral radicals formed by proton loss from the radical cations of linear carotenoids has been examined as a function of conjugation length from n = 15 to 9. For a maximum conjugation length of n = 15 (bisdehydrolycopene, a symmetrical compound), proton loss can occur from any of the 10 methyl groups, with proton loss from the methyl group at position C1 or C1' being the most favorable. In contrast, the most energetically favorable proton loss from the radical cations of lycopene, neurosporene, spheroidene, spheroidenone, spirilloxanthin, and anhydrorhodovibrin occurs from methylene groups that extend from the conjugated system. For example, decreasing the conjugation length to n = 11 (lycopene) by saturation of the double bonds C3-C4 and at C3'-C4' of bisdehydrolycopene favors proton loss at C4 or C4' methylene groups. Saturation at C7'-C8' in the case of neurosporene, spheroidene, and spheroidenone (n = 9, 10, 11) favors the formation of a neutral radical at the C8' methylene group. Saturation of C1-C2 by addition of a methoxy group to a bisdehydrolycopene-like structure with conjugation of n = 12 or 13 (anhydrorhodovibrin, spirilloxanthin) favors proton loss at the C2 methylene group. As a consequence of deprotonation of the radical cation, the unpaired electron spin distribution changes so that larger β-methyl proton couplings occur for the neutral radicals (13-16 MHz) than for the radical cation (7-10 MHz), providing a means to identify possible carotenoid radicals in biological systems by Mims ENDOR.
Figures










References
-
- Koepke J, Hu X, Muenke C, Schulten K, Michel H. Structure. 1996;4:581–97. - PubMed
-
- Qian P, Saiki K, Mizoguchi T, Hara K, Sashima T, Fujii R, Koyama Y. Photochem Photobiol. 2001;74:444–52. - PubMed
-
- Polivka T, Pullerits T, Frank HA, Cogdell RJ, Sundstrom V. J Phys Chem B. 2004;108:15398–15407.
-
- Wormit M, Harbach PH, Mewes JM, Amarie S, Wachtveitl J, Dreuw A. Biochim Biophys Acta. 2009;1787:738–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

