TBP-related factors: a paradigm of diversity in transcription initiation
- PMID: 21711503
- PMCID: PMC3142196
- DOI: 10.1186/2045-3701-1-23
TBP-related factors: a paradigm of diversity in transcription initiation
Abstract
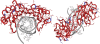
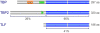
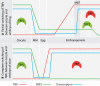
TATA binding protein (TBP) is a key component of the eukaryotic transcription initiation machinery. It functions in several complexes involved in core promoter recognition and assembly of the pre-initiation complex. Through gene duplication eukaryotes have expanded their repertoire of TATA binding proteins, leading to a variable composition of the transcription machinery. In vertebrates this repertoire consists of TBP, TBP-like factor (TLF, also known as TBPL1, TRF2) and TBP2 (also known as TBPL2, TRF3). All three factors are essential, with TLF and TBP2 playing important roles in development and differentiation, in particular gametogenesis and early embryonic development, whereas TBP dominates somatic cell transcription. TBP-related factors may compete for promoters when co-expressed, but also show preferential interactions with subsets of promoters. Initiation factor switching occurs on account of differential expression of these proteins in gametes, embryos and somatic cells. Paralogs of TFIIA and TAF subunits account for additional variation in the transcription initiation complex. This variation in core promoter recognition accommodates the expanded regulatory capacity and specificity required for germ cells and embryonic development in higher eukaryotes.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

