Thermal conductivity and viscosity measurements of ethylene glycol-based Al2O3 nanofluids
- PMID: 21711737
- PMCID: PMC3211279
- DOI: 10.1186/1556-276X-6-221
Thermal conductivity and viscosity measurements of ethylene glycol-based Al2O3 nanofluids
Abstract
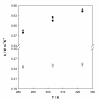
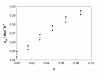
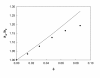
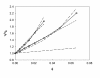
The dispersion and stability of nanofluids obtained by dispersing Al2O3 nanoparticles in ethylene glycol have been analyzed at several concentrations up to 25% in mass fraction. The thermal conductivity and viscosity were experimentally determined at temperatures ranging from 283.15 K to 323.15 K using an apparatus based on the hot-wire method and a rotational viscometer, respectively. It has been found that both thermal conductivity and viscosity increase with the concentration of nanoparticles, whereas when the temperature increases the viscosity diminishes and the thermal conductivity rises. Measured enhancements on thermal conductivity (up to 19%) compare well with literature values when available. New viscosity experimental data yield values more than twice larger than the base fluid. The influence of particle size on viscosity has been also studied, finding large differences that must be taken into account for any practical application. These experimental results were compared with some theoretical models, as those of Maxwell-Hamilton and Crosser for thermal conductivity and Krieger and Dougherty for viscosity.
Figures


References
-
- Smalley RE. Future global energy prosperity: The terawatt challenge. MRS Bull. 2005;30:412. doi: 10.1557/mrs2005.124. - DOI
-
- Wen DS, Lin G, Vafaei S, Zhang K. Review of nanofluids for heat transfer applications. Particuology. 2009;7:141. doi: 10.1016/j.partic.2009.01.007. - DOI
-
- Wen DS. Nanofuel as a potential secondary energy carrier. Energy Environ Sci. 2010;3:591. doi: 10.1039/b906384f. - DOI
-
- Das SK, Choi SUS, Yu W, Pradeep T. Nanofluids: Science and Technology. New York: Wiley; 2008.
-
- Choi SUS, Zhang ZG, Keblinski P. In: Encyclopedia of Nanoscience and Nanotechnology, 6. Nalwa SH, editor. New York: Scientific Publishers; 2004. Nanofluids; pp. 757–773.
LinkOut - more resources
Full Text Sources

