Polytetrafluorethylene-Au as a substrate for surface-enhanced Raman spectroscopy
- PMID: 21711893
- PMCID: PMC3211456
- DOI: 10.1186/1556-276X-6-366
Polytetrafluorethylene-Au as a substrate for surface-enhanced Raman spectroscopy
Abstract
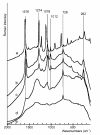
This study deals with preparation of substrates suitable for surface-enhanced Raman spectroscopy (SERS) applications by sputtering deposition of gold layer on the polytetrafluorethylene (PTFE) foil. Time of sputtering was investigated with respect to the surface properties. The ability of PTFE-Au substrates to enhance Raman signals was investigated by immobilization of biphenyl-4,4'-dithiol (BFD) from the solutions with various concentrations. BFD was also used for preparation of sandwich structures with Au or Ag nanoparticles by two different procedures. Results showed that PTFE can be used for fabrication of SERS active substrate with easy handle properties at low cost. This substrate was sufficient for the measurement of SERS spectrum of BFD even at 10-8 mol/l concentration.
Figures


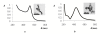

References
-
- Moskovits M. Surface-enhanced spectroscopy. Rev Mod Phys. 1985;57:783. doi: 10.1103/RevModPhys.57.783. - DOI
-
- Campion A, Kambhampati P. Surface-enhanced Raman scattering. Chem Soc Rev. 1998;27:241. doi: 10.1039/a827241z. - DOI
-
- Sant'Ana AC, Rocha TCR, Santos PS, Zanchet D, Temperini MLA. Size-dependent SERS enhancement of colloidal silver nanoplates: the case of 2-amino-5-nitropyridine. J Raman Spectrosc. 2009;40:183. doi: 10.1002/jrs.2103. - DOI
-
- Jeanmaire DL, Van Duyne RP. Surface Raman spectroelectrochemistry Part I. heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J Electroanal Chem. 1977;84:1. doi: 10.1016/S0022-0728(77)80224-6. - DOI
-
- Albrecht MG, Creighton JA. Anomalously intense Raman spectra of pyridine at a silver electrode. J Am Chem Soc. 1977;99:5215. doi: 10.1021/ja00457a071. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

