Size-dependent catalytic and melting properties of platinum-palladium nanoparticles
- PMID: 21711923
- PMCID: PMC3211490
- DOI: 10.1186/1556-276X-6-396
Size-dependent catalytic and melting properties of platinum-palladium nanoparticles
Abstract
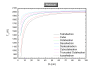
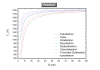
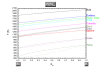
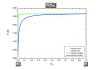
While nanocatalysis is a very active field, there have been very few studies in the size/shape-dependent catalytic properties of transition metals from a thermodynamical approach. Transition metal nanoparticles are very attractive due their high surface to volume ratio and their high surface energy. In particular, in this paper we focus on the Pt-Pd catalyst which is an important system in catalysis. The melting temperature, melting enthalpy, and catalytic activation energy were found to decrease with size. The face centered cubic crystal structure of platinum and palladium has been considered in the model. The shape stability has been discussed. The phase diagram of different polyhedral shapes has been plotted and the surface segregation has been considered. The model predicts a nanoparticle core rich in Pt surrounded by a layer enriched in Pd. The Pd segregation at the surface strongly modifies the catalytic activation energy compared to the non-segregated nanoparticle. The predictions were compared with the available experimental data in the literature. PACS: 65.80-g; 82.60.Qr; 64.75.Jk.
Figures

References
-
- Maye MM, Kariuki NN, Luo J, Han L, Njoki P, Wang L, Lin Y, Naslund HR, Zhong CJ. Electrocatalytic reduction of oxygen: gold and gold-platinum nanoparticle catalysts prepared by two phase protocol. Gold Bulletin. 2004;37:217. doi: 10.1007/BF03215216. - DOI
-
- Deng L, Hu W, Deng H, Xiao S. Surface segregation and structural features of bi-metallic Au-Pt nanoparticles. Journal of Physical Chemistry C. 2010;114:11026. doi: 10.1021/jp100194p. - DOI
-
- Sealy C. The problem with platinum. Materials Today. 2008;11:65–68. doi: 10.1016/S1369-7021(08)70254-2. - DOI
-
- Guisbiers G, Buchaillot L. Universal size/shape-dependent law for characteristic temperatures. Physics Letters A. 2009;374:305–308. doi: 10.1016/j.physleta.2009.10.054. - DOI
LinkOut - more resources
Full Text Sources

