Ammonium perfluorooctanoate may cause testosterone reduction by adversely affecting testis in relation to PPARα
- PMID: 21712084
- PMCID: PMC6661110
- DOI: 10.1016/j.toxlet.2011.06.015
Ammonium perfluorooctanoate may cause testosterone reduction by adversely affecting testis in relation to PPARα
Abstract
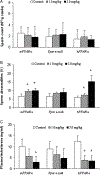
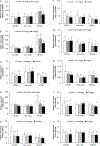
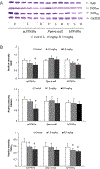
Perfluorooctanoate, a peroxisome proliferator-activated receptor alpha (PPARα) agonist, has the potential to lower testosterone levels as a result of testicular toxicity. To elucidate the mechanism and impact of PPARα on this reproductive toxicity, ammonium perfluorooctanoate (APFO) at doses of 0, 1.0 (low) mg/kg/day, or 5.0 (high) mg/kg/day was orally given daily to 129/sv wild-type (mPPARα), Pparα-null and PPARα-humanized (hPPARα) mice for 6 weeks. Both low- and high-dose APFO significantly reduced plasma testosterone concentrations in mPPARα and hPPARα mice, respectively. These decreases may, in part, be associated with decreased expression of mitochondrial cytochrome P450 side-chain cleavage enzyme, steroidogenic acute regulatory protein or peripheral benzodiazepine receptor as well as microsomal cytochrome P450(17α) involved in the steroidogenesis. Additionally, both doses increased abnormalities in sperm morphology and vacuolated cells in the seminiferous tubules of both mouse lines. In contrast, APFO caused only a marginal effect either on the testosterone synthesis system or sperm and testis morphology in Pparα-null mice. These results suggest that APFO may disrupt testosterone biosynthesis by lowering the delivery of cholesterol into the mitochondria and decreasing the conversion of cholesterol to pregnenolone and androstandione in the testis of mPPARα and hPPARα mice, which may, in part, be related to APFO-induced mitochondrial damage.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
None declared.
Figures

Similar articles
-
Microgram-order ammonium perfluorooctanoate may activate mouse peroxisome proliferator-activated receptor alpha, but not human PPARalpha.Toxicology. 2009 Nov 9;265(1-2):27-33. doi: 10.1016/j.tox.2009.09.004. Epub 2009 Sep 12. Toxicology. 2009. PMID: 19751795 Free PMC article.
-
Modulation of ammonium perfluorooctanoate-induced hepatic damage by genetically different PPARα in mice.Arch Toxicol. 2012 Jan;86(1):63-74. doi: 10.1007/s00204-011-0704-3. Epub 2011 Apr 17. Arch Toxicol. 2012. PMID: 21499893 Free PMC article.
-
The role of mouse and human peroxisome proliferator-activated receptor-α in modulating the hepatic effects of perfluorooctane sulfonate in mice.Toxicology. 2022 Jan 15;465:153056. doi: 10.1016/j.tox.2021.153056. Epub 2021 Nov 30. Toxicology. 2022. PMID: 34861291 Free PMC article.
-
Hepatic peroxisome proliferator-activated receptor α may have an important role in the toxic effects of di(2-ethylhexyl)phthalate on offspring of mice.Toxicology. 2011 Oct 28;289(1):1-10. doi: 10.1016/j.tox.2011.02.007. Epub 2011 Feb 24. Toxicology. 2011. PMID: 21354252
-
Pathology Working Group review and evaluation of proliferative lesions of mammary gland tissues in female rats fed ammonium perfluorooctanoate (APFO) in the diet for 2 years.Drug Chem Toxicol. 2010 Apr;33(2):131-7. doi: 10.3109/01480541003667610. Drug Chem Toxicol. 2010. PMID: 20307141
Cited by
-
PFOA-Induced Ovotoxicity Differs Between Lean and Obese Mice With Impacts on Ovarian Reproductive and DNA Damage Sensing and Repair Proteins.Toxicol Sci. 2022 Nov 23;190(2):173-188. doi: 10.1093/toxsci/kfac104. Toxicol Sci. 2022. PMID: 36214631 Free PMC article.
-
Peroxisome proliferator-activated receptor gamma signaling in human sperm physiology.Asian J Androl. 2015 Nov-Dec;17(6):942-7. doi: 10.4103/1008-682X.150253. Asian J Androl. 2015. PMID: 25851655 Free PMC article. Review.
-
Targeting testis-specific proteins to inhibit spermatogenesis: lesson from endocrine disrupting chemicals.Expert Opin Ther Targets. 2013 Jul;17(7):839-55. doi: 10.1517/14728222.2013.791679. Epub 2013 Apr 22. Expert Opin Ther Targets. 2013. PMID: 23600530 Free PMC article. Review.
-
Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food.EFSA J. 2018 Dec 13;16(12):e05194. doi: 10.2903/j.efsa.2018.5194. eCollection 2018 Dec. EFSA J. 2018. PMID: 32625773 Free PMC article.
-
Implications of adiponectin in linking metabolism to testicular function.Endocrine. 2014 May;46(1):16-28. doi: 10.1007/s12020-013-0102-0. Epub 2013 Nov 28. Endocrine. 2014. PMID: 24287788 Review.
References
-
- Abbott BD, Wolf CJ, Schmid JE, Das KP, Zehr RD, Helfant L, Nakayama S, Lindstrom AB, Strynar MJ, Lau C, 2007. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferatoractivated receptor-alpha. Toxicol. Sci. 98,571–581. - PubMed
-
- Biegel LB, Liu RCM, Hurtt ME, Cook JC, 1995. Effects of ammonium perfluo-rooctanoate on Leydig-cell function: invitro, invivo, and exvivostudies.Toxicol. Appl. Pharmacol. 134,18–25. - PubMed
-
- Bryan JH,1970Aneosin-fastgreen-naphtholyellowmixturefordifferentialstaining of cytologic components in mammalian spermatozoa. Stain Technol. 45, 231–236. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

