2',5'-Dihydroxychalcone-induced glutathione is mediated by oxidative stress and kinase signaling pathways
- PMID: 21712085
- PMCID: PMC3257860
- DOI: 10.1016/j.freeradbiomed.2011.05.041
2',5'-Dihydroxychalcone-induced glutathione is mediated by oxidative stress and kinase signaling pathways
Abstract
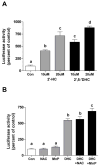
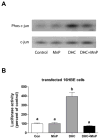
Hydroxychalcones are naturally occurring compounds that continue to attract considerable interest because of their anti-inflammatory and antiangiogenic properties. They have been reported to inhibit the synthesis of the inducible nitric oxide synthase and to induce the expression of heme oxygenase-1. This study examines the mechanisms by which 2',5'-dihydroxychalcone (2',5'-DHC) induces an increase in cellular glutathione (GSH) levels using a cell line stably expressing a luciferase reporter gene driven by antioxidant-response elements (MCF-7/AREc32). The 2',5'-DHC-induced increase in cellular GSH levels was partially inhibited by the catalytic antioxidant MnTDE-1,3-IP(5+), suggesting that reactive oxygen species (ROS) mediate the antioxidant adaptive response. 2',5'-DHC treatment induced phosphorylation of the c-Jun N-terminal kinase (JNK) pathway, which was also inhibited by MnTDE-1,3-IP(5+). These findings suggest a ROS-dependent activation of the AP-1 transcriptional response. However, whereas 2',5'-DHC triggered the NF-E2-related factor 2 (Nrf2) transcriptional response, cotreatment with MnTDE-1,3-IP(5+) did not decrease 2',5'-DHC-induced Nrf2/ARE activity, showing that this pathway is not dependent on ROS. Moreover, pharmacological inhibitors of mitogen-activated protein kinase (MAPK) pathways showed a role for JNK and p38MAPK in mediating the 2',5'-DHC-induced Nrf2 response. These findings suggest that the 2',5'-DHC-induced increase in GSH levels results from a combination of ROS-dependent and ROS-independent pathways.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway.Cell Signal. 2014 Mar;26(3):512-20. doi: 10.1016/j.cellsig.2013.11.029. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308969
-
Miltirone protects human EA.hy926 endothelial cells from oxidized low-density lipoprotein-derived oxidative stress via a heme oxygenase-1 and MAPK/Nrf2 dependent pathway.Phytomedicine. 2016 Dec 15;23(14):1806-1813. doi: 10.1016/j.phymed.2016.11.003. Epub 2016 Nov 4. Phytomedicine. 2016. PMID: 27912883
-
Humic acid in drinking well water induces inflammation through reactive oxygen species generation and activation of nuclear factor-κB/activator protein-1 signaling pathways: a possible role in atherosclerosis.Toxicol Appl Pharmacol. 2014 Jan 15;274(2):249-62. doi: 10.1016/j.taap.2013.11.002. Epub 2013 Nov 12. Toxicol Appl Pharmacol. 2014. PMID: 24239652
-
2,3,5,6-Tetramethylpyrazine (TMP) down-regulated arsenic-induced heme oxygenase-1 and ARS2 expression by inhibiting Nrf2, NF-κB, AP-1 and MAPK pathways in human proximal tubular cells.Arch Toxicol. 2016 Sep;90(9):2187-2200. doi: 10.1007/s00204-015-1600-z. Epub 2015 Sep 24. Arch Toxicol. 2016. PMID: 26404762 Free PMC article.
-
Lannea coromandelica (Houtt.) Merr. Induces Heme Oxygenase 1 (HO-1) Expression and Reduces Oxidative Stress via the p38/c-Jun N-Terminal Kinase-Nuclear Factor Erythroid 2-Related Factor 2 (p38/JNK-NRF2)-Mediated Antioxidant Pathway.Int J Mol Sci. 2017 Jan 29;18(2):266. doi: 10.3390/ijms18020266. Int J Mol Sci. 2017. PMID: 28146074 Free PMC article.
Cited by
-
β-TrCP1 Is a Vacillatory Regulator of Wnt Signaling.Cell Chem Biol. 2017 Aug 17;24(8):944-957.e7. doi: 10.1016/j.chembiol.2017.06.009. Epub 2017 Jul 20. Cell Chem Biol. 2017. PMID: 28736239 Free PMC article.
-
A synthetic chalcone as a potent inducer of glutathione biosynthesis.J Med Chem. 2012 Feb 9;55(3):1382-8. doi: 10.1021/jm2016073. Epub 2012 Jan 30. J Med Chem. 2012. PMID: 22239485 Free PMC article.
-
MDA-7/IL-24 inhibits Nrf2-mediated antioxidant response through activation of p38 pathway and inhibition of ERK pathway involved in cancer cell apoptosis.Cancer Gene Ther. 2014 Oct;21(10):416-26. doi: 10.1038/cgt.2014.45. Epub 2014 Sep 19. Cancer Gene Ther. 2014. PMID: 25236495
-
Antioxidant therapeutics: Pandora's box.Free Radic Biol Med. 2014 Jan;66:58-64. doi: 10.1016/j.freeradbiomed.2013.05.047. Epub 2013 Jul 12. Free Radic Biol Med. 2014. PMID: 23856377 Free PMC article. Review.
-
Discovery of 4-Anilinoquinolinylchalcone Derivatives as Potential NRF2 Activators.Molecules. 2020 Jul 8;25(14):3133. doi: 10.3390/molecules25143133. Molecules. 2020. PMID: 32650607 Free PMC article.
References
-
- Lawrence NJ, McGown AT. The chemistry and biology of antimitotic chalcones and related enone systems. Curr Pharm Des. 2005;11:1679–1693. - PubMed
-
- Dimmock JR, Elias DW, Beazely MA, Kandepu NM. Bioactivities of chalcones. Curr Med Chem. 1999;6:1125–1149. - PubMed
-
- Go ML, Wu X, Liu XL. Chalcones: an update on cytotoxic and chemoprotective properties. Curr Med Chem. 2005;12:481–499. - PubMed
-
- Cheng JH, Hung CF, Yang SC, Wang JP, Won SJ, Lin CN. Synthesis and cytotoxic, anti-inflammatory, and anti-oxidant activities of 2′,5′-dialkoxylchalcones as cancer chemopreventive agents. Bioorg Med Chem. 2008;16:7270–7276. - PubMed
-
- Nam NH, Kim Y, You YJ, Hong DH, Kim HM, Ahn BZ. Cytotoxic 2′,5′-dihydroxychalcones with unexpected antiangiogenic activity. Eur J Med Chem. 2003;38:179–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

