Molecular in situ topology of Aczonin/Piccolo and associated proteins at the mammalian neurotransmitter release site
- PMID: 21712437
- PMCID: PMC3150911
- DOI: 10.1073/pnas.1101707108
Molecular in situ topology of Aczonin/Piccolo and associated proteins at the mammalian neurotransmitter release site
Abstract
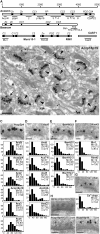
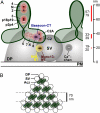
The protein machinery of neurotransmitter exocytosis requires efficient orchestration in space and time, for speed and precision of neurotransmission and also for synaptic ontogeny and plasticity. However, its spatial organization in situ is virtually unknown. Aczonin/Piccolo is a putative organizer protein of mammalian active zones. We determined by immunogold electron microscopy (EM) (i) the spatial arrangement (i.e., topology) of 11 segments of the Aczonin polypeptide in situ, and correlated it to (ii) the positioning of Aczonin-interacting domains of Bassoon, CAST/ELKS, Munc13, and RIM and (iii) the ultrastructurally defined presynaptic macromolecular aggregates known as dense projections and synaptic ribbons. At conventional synapses, Aczonin assumes a compact molecular topology within a layer 35 to 80 nm parallel to the plasma membrane (PM), with a "trunk" sitting on the dense projection top and a C-terminal "arm" extending down toward the PM and sideward to the dense projection periphery. At ribbon synapses, Aczonin occupies the whole ribbon area. Bassoon colocalizes with Aczonin at conventional synapses but not at ribbon synapses. At both conventional and ribbon synapses, CAST, Munc13, and RIM are segregated from Aczonin, closer to the PM, and Aczonin is positioned such that it may control the access of neurotransmitter vesicles to the fusion site.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wojcik SM, Brose N. Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron. 2007;55:11–24. - PubMed
-
- Schoch S, Gundelfinger ED. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006;326:379–391. - PubMed
-
- Phillips GR, et al. The presynaptic particle web: Ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. - PubMed
-
- Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 2004;19:262–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

