Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid
- PMID: 21712440
- PMCID: PMC3141957
- DOI: 10.1073/pnas.1102664108
Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid
Abstract
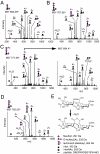
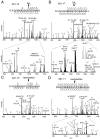
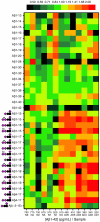
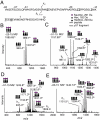
The proteolytic processing of human amyloid precursor protein (APP) into shorter aggregating amyloid β (Aβ)-peptides, e.g., Aβ1-42, is considered a critical step in the pathogenesis of Alzheimer's disease (AD). Although APP is a well-known membrane glycoprotein carrying both N- and O-glycans, nothing is known about the occurrence of released APP/Aβ glycopeptides in cerebrospinal fluid (CSF). We used the 6E10 antibody and immunopurified Aβ peptides and glycopeptides from CSF samples and then liquid chromatography-tandem mass spectrometry for structural analysis using collision-induced dissociation and electron capture dissociation. In addition to 33 unglycosylated APP/Aβ peptides, we identified 37 APP/Aβ glycopeptides with sialylated core 1 like O-glycans attached to Thr(-39, -21, -20, and -13), in a series of APP/AβX-15 glycopeptides, where X was -63, -57, -52, and -45, in relation to Asp1 of the Aβ sequence. Unexpectedly, we also identified a series of 27 glycopeptides, the Aβ1-X series, where X was 20 (DAEFRHDSGYEVHHQKLVFF), 19, 18, 17, 16, and 15, which were all uniquely glycosylated on Tyr10. The Tyr10 linked O-glycans were (Neu5Ac)(1-2)Hex(Neu5Ac)HexNAc-O- structures with the disialylated terminals occasionally O-acetylated or lactonized, indicating a terminal Neu5Acα2,8Neu5Ac linkage. We could not detect any glycosylation of the Aβ1-38/40/42 isoforms. We observed an increase of up to 2.5 times of Tyr10 glycosylated Aβ peptides in CSF in six AD patients compared to seven non-AD patients. APP/Aβ sialylated O-glycans, including that of a Tyr residue, the first in a mammalian protein, may modulate APP processing, inhibiting the amyloidogenic pathway associated with AD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sato Y, et al. Study of the sugar chains of recombinant human amyloid precursor protein produced by Chinese hamster ovary cells. Biochim Biophys Acta. 1999;1472:344–358. - PubMed
-
- Akasaka-Manya K, et al. Increased bisecting and core-fucosylated N-glycans on mutant human amyloid precursor proteins. Glycoconjugate J. 2008;25:775–786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

