A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans
- PMID: 21712812
- PMCID: PMC3160189
- DOI: 10.1038/emboj.2011.215
A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans
Abstract
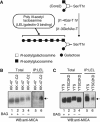
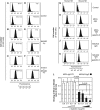
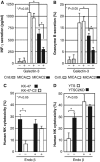
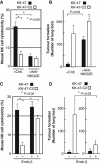
The O-glycan branching enzyme, core2 β-1,6-N-acetylglucosaminyltransferase (C2GnT), forms O-glycans containing an N-acetylglucosamine branch connected to N-acetylgalactosamine (core2 O-glycans) on cell-surface glycoproteins. Here, we report that upregulation of C2GnT is closely correlated with progression of bladder tumours and that C2GnT-expressing bladder tumours use a novel strategy to increase their metastatic potential. Our results showed that C2GnT-expressing bladder tumour cells are highly metastatic due to their high ability to evade NK cell immunity and revealed the molecular mechanism of the immune evasion by C2GnT expression. Engagement of an NK-activating receptor, NKG2D, by its tumour-associated ligand, Major histocompatibility complex class I-related chain A (MICA), is critical to tumour rejection by NK cells. In C2GnT-expressing bladder tumour cells, poly-N-acetyllactosamine was present on core2 O-glycans on MICA, and galectin-3 bound the NKG2D-binding site of MICA through this poly-N-acetyllactosamine. Galectin-3 reduced the affinity of MICA for NKG2D, thereby severely impairing NK cell activation and silencing the NK cells. This new mode of NK cell silencing promotes immune evasion of C2GnT-expressing bladder tumour cells, resulting in tumour metastasis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Core2 O-glycan-expressing prostate cancer cells are resistant to NK cell immunity.Mol Med Rep. 2013 Feb;7(2):359-64. doi: 10.3892/mmr.2012.1189. Epub 2012 Nov 19. Mol Med Rep. 2013. PMID: 23165940 Free PMC article.
-
MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis.Int J Oncol. 2012 Jun;40(6):1831-8. doi: 10.3892/ijo.2012.1411. Epub 2012 Mar 23. Int J Oncol. 2012. PMID: 22446589 Free PMC article.
-
A mechanism for evasion of CTL immunity by altered O-glycosylation of HLA class I.J Biochem. 2017 Jun 1;161(6):479-492. doi: 10.1093/jb/mvw096. J Biochem. 2017. PMID: 28011817
-
Tumor defense systems using O-glycans.Biol Pharm Bull. 2012;35(10):1633-6. doi: 10.1248/bpb.b12-00367. Biol Pharm Bull. 2012. PMID: 23037152 Review.
-
Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications.Scand J Immunol. 2013 Aug;78(2):120-9. doi: 10.1111/sji.12072. Scand J Immunol. 2013. PMID: 23679194 Review.
Cited by
-
Behavior of blood plasma glycan features in bladder cancer.PLoS One. 2018 Jul 24;13(7):e0201208. doi: 10.1371/journal.pone.0201208. eCollection 2018. PLoS One. 2018. PMID: 30040854 Free PMC article.
-
Understanding Tricky Cellular and Molecular Interactions in Pancreatic Tumor Microenvironment: New Food for Thought.Front Immunol. 2022 May 31;13:876291. doi: 10.3389/fimmu.2022.876291. eCollection 2022. Front Immunol. 2022. PMID: 35711414 Free PMC article. Review.
-
Molecular Basis and Role of Siglec-7 Ligand Expression on Chronic Lymphocytic Leukemia B Cells.Front Immunol. 2022 May 31;13:840388. doi: 10.3389/fimmu.2022.840388. eCollection 2022. Front Immunol. 2022. PMID: 35711441 Free PMC article.
-
Galectin-3 and cancer immunotherapy: a glycobiological rationale to overcome tumor immune escape.J Exp Clin Cancer Res. 2024 Feb 6;43(1):41. doi: 10.1186/s13046-024-02968-2. J Exp Clin Cancer Res. 2024. PMID: 38317202 Free PMC article.
-
Galectin-3, Possible Role in Pathogenesis of Periodontal Diseases and Potential Therapeutic Target.Front Pharmacol. 2021 Mar 19;12:638258. doi: 10.3389/fphar.2021.638258. eCollection 2021. Front Pharmacol. 2021. PMID: 33815121 Free PMC article. Review.
References
-
- Abdou AG, Hammam MA, Farargy SE, Farag AG, El Shafey EN, Farouk S, Elnaidany NF (2010) Diagnostic and prognostic role of galectin 3 expression in cutaneous melanoma. Am J Dermatopathol 32: 809–814 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

